一种有效抑制或杀灭多重耐药细菌的化合物及其制备方法与应用与流程
本发明属于医药领域,具体涉及一种有效抑制或杀灭多重耐药细菌的化合物及其制备方法与应用。
背景技术:
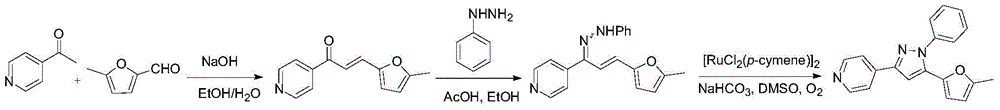
:抗生素(antibiotics)是指某些细菌、放线菌、真菌等微生物的次级代谢产物,或用化学方法合成的相同化合物或结构类似物,在低浓度下对各种病原性微生物或肿瘤细胞有强力杀灭、抑制作用或有其他药理作用的药物。目前已知天然抗生素不下万种。中国是抗生素使用大国,也是抗生素生产大国。由于抗生素的大量使用使得中国成为世界上滥用抗生素问题最严重的国家之一,并且导致许多耐药性病菌的出现,如超级细菌等。合成抗菌药物是指除抗生素以外的抗菌化合物,如喹诺酮类抗菌药,磺胺类药物、恶唑烷酮类、异喹啉类抗菌药等。但由于这些化合物的长期使用也出现了耐药菌。基于此,开发结构新颖并且作用机制独特的抗菌药物对于杀灭或抑制广谱耐药菌的生长具有非常重要的意义。目前出现的较为严重的耐药菌包括:耐甲氧西林金黄色葡萄球菌MRSA、耐万古霉素金黄色葡萄球菌VRSA、耐喹诺酮金黄色葡萄球菌QRSA、耐青霉素肺炎链球菌PRSP、耐万古霉素粪肠球菌VRE、以及多重耐药的革兰氏阴性菌等。通过高通量筛选等方法快速找到针对以上耐药菌株的小分子苗头化合物,并进行结构优化得到先导化合物,进而得到药物候选物及最终成药是我们一直追求的目标。同时发展小分子探针,找到可用的新靶点也是我们不懈的追求。技术实现要素:本发明的目的是提供一类能有效抑制或杀灭细菌类微生物的化合物。本发明所提供的化合物,其结构通式如式I所示:式I中的虚线表示R2所连接的碳原子与R3所连接的碳原子之间通过单键或双键连接;式I中,R1、R2、R3、R4具体选自如下所示的取代基:式I中,R1、R2、R3、R4进一步优选自如下所示的取代基:式I中,R1、R2、R3、R4最优选自如下所示的取代基:上述式I所示化合物药学上可接受的盐也属于本发明的保护范围。其中,所述盐为无机酸盐或有机酸盐。所述无机酸盐选自下述任意一种无机酸形成的盐:盐酸、硫酸和磷酸。所述有机酸盐选自下述任意一种有机酸形成的盐:乙酸、三氟乙酸、丙二酸、柠檬酸和对甲苯磺酸。本发明还提供了上述式I化合物的制备方法。所述式I化合物的制备方法根据取代基的不同,分为下述几类:当R1为含氮芳香化合物(如吡啶类化合物、吡嗪类化合物)时,采用如下方式制备:式II与式Ⅲ化合物进行羟醛缩合反应生成式Ⅳ化合物;式Ⅳ化合物与取代肼发生脱水缩合反应生成式Ⅴ化合物,式Ⅴ化合物发生分子内碳氮偶联反应生成终产物。当R1为苯环或取代苯环时,采用如下方式:式II与式Ⅲ化合物进行羟醛缩合反应生成式Ⅳ化合物;式Ⅳ化合物与取代肼发生脱水缩合反应并分子内关环生成式Ⅴ化合物,式Ⅴ化合物发生氧化芳香化生成终产物。其中,R1或R3为呋喃或邻甲基呋喃,可通过氧化开环反应生成顺式或反式不饱和1,4-二酮产物。此中间体可通过还原、过氧化等进一步转化。具体说明如下:当式I中,R1、R2、R3、R4选自如下所示的取代基:且式I中的R2所连接的碳原子与R3所连接的碳原子之间通过双键连接时,所述式I化合物(具体如D3,D3-1到D3-18,D3-37)的制备方法包括下述步骤:1)将式II所示的化合物与式Ⅲ所示的化合物进行羟醛缩合反应,得到式Ⅳ所示的化合物;式II式Ⅲ式Ⅳ式Ⅴ2)式Ⅳ所示的化合物与R4NHNH2进行脱水缩合反应,得到式Ⅴ所示的化合物;3)式Ⅴ所示的化合物进行偶联反应,得到式I化合物。所述步骤1)中,所述式II所示的化合物与式Ⅲ所示的化合物的摩尔比为1:1。所述步骤1)中,所述羟醛缩合反应在碱的催化下进行,所述碱具体可为氢氧化钠或氢氧化钾,所述碱的用量为式II所示的化合物与式Ⅲ所示的化合物的摩尔总量的1.1倍。所述羟醛缩合反应在乙醇水溶液中进行,所述反应的反应温度为室温,反应时间为1-6h。所述步骤2)中,所述式Ⅳ所示的化合物与R4NHNH2的摩尔比为1:1.2-1.5。所投肼的量为一个范围,主要是使得不饱和醛酮转化完全。所述步骤2)中,所述脱水缩合反应在醋酸或质量浓度为98%的浓硫酸的催化下进行。所述醋酸或浓硫酸的用量为底物(不饱和酮)摩尔量的0.3倍。所述脱水缩合反应于乙醇中回流2-6h。所述步骤3)中,所述偶联反应在钌催化剂及碳酸氢钠存在下进行。所述钌催化剂具体可为对伞花烃二氯化钌二聚体,分子式:[RuCl2(p-cymene)]2。所述钌催化剂的用量为底物不饱和腙摩尔量的5%;所述碳酸氢钠的用量为底物不饱和腙摩尔量的1倍。所述偶联反应于二甲基亚砜溶液中100-110℃反应12-24h。当式I中,R1、R2、R3、R4选自如下所示的取代基:且式I中的R2所连接的碳原子与R3所连接的碳原子之间通过单键连接时,所述式I化合物的制备方法包括下述步骤:a)将式II所示的化合物与式Ⅲ所示的化合物进行羟醛缩合反应,得到式Ⅳ所示的化合物;b)式Ⅳ所示的化合物与R4NHNH2或R4NHNH2·HCl进行脱水缩合和分子内1,4-加成反应,得到式Ⅵ所示的化合物(即式I所示的化合物)。所述步骤a)中,所述式II所示的化合物与式Ⅲ所示的化合物的摩尔比为1:1。所述步骤a)中,所述羟醛缩合反应在碱的催化下进行,所述碱具体可为氢氧化钠或氢氧化钾,所述碱的用量为式II所示的化合物与式Ⅲ所示的化合物的摩尔总量的1.1倍。所述羟醛缩合反应在乙醇水溶液中进行,所述反应的反应温度为室温,反应时间为1-6h。所述步骤b)中,所述式Ⅳ所示的化合物与R4NHNH2的摩尔比为1:1.2-1.5。所投肼的量为一个范围,主要是使得不饱和醛酮转化完全。所述步骤b)中的反应条件为在浓硫酸(质量浓度为98%)催化下于甲醇溶液中回流4-6h。所述浓硫酸的加入量为每摩尔底物(不饱和酮)加入0.2mL所述浓硫酸。当式I中,R1、R2、R3、R4选自如下所示的取代基:且式I中的R2所连接的碳原子与R3所连接的碳原子之间通过双键连接时,所述式I化合物的制备方法包括下述步骤:在酸性条件下,使式Ⅵ所示的化合物进行氧化芳香化反应,得到式I化合物。所述反应条件为:在Pd催化下,于醋酸溶液中氧气氛围下90-100℃搅拌8-12h。所述Pd催化剂的用量为底物吡唑啉质量的30%。所述Pd催化剂具体可为Pd/C催化剂或Pd(OAc)2.所述醋酸溶液的浓度为0.2-0.5mol/L。当R3为所述式I化合物的制备方法包括下述步骤:将R3为5-甲基呋喃或5-乙基呋喃的式I化合物进行氧化开环反应,得到如下所示的化合物。所述反应的反应条件为:在二氯甲烷中与间氯过氧苯甲酸(m-CPBA)室温搅拌1h;所述R3为5-甲基呋喃或5-乙基呋喃的式I化合物与间氯过氧苯甲酸摩尔比为1:2.5-3.0。当R3为所述式I化合物的制备方法包括下述步骤:将R3为5-甲基呋喃或5-乙基呋喃的的式I化合物进行氧化开环反应,得到如下所示的化合物;所述反应的反应条件为:1)二氯甲烷中与2.5-3.0equiv的间氯过氧苯甲酸室温搅拌1h。此条件下生成的反式产物为次要产物(主要生成顺式产物);2)二氯乙烷、醋酸、水的混合溶剂中与过硫酸钾或过硫酸铵加热搅拌12h,此条件下产率中等,约为40%-50%;3)式I化合物中5-顺式二烯酮的产物在碘单质存在下乙醚溶液中回流12h可以生成高产率的5-反式二烯酮产物。当R3为所述式I化合物的制备方法包括下述步骤:将R3为5-甲基呋喃或5-顺式二烯酮的式I化合物进行氧化开环及过氧化反应或过氧化反应(根据不同底物控制氧化剂的量),得到如下所示的化合物。所述反应的反应条件为R3为5-甲基呋喃的式I化合物在二氯甲烷中与大于4.0equiv间氯过氧苯甲酸反应生成高产率过氧化产物;或R3为5-顺式二烯酮的式I化合物在二氯甲烷中与1.5equiv间氯过氧苯甲酸反应生成高产率过氧化产物。当R2为溴、碘时,所述式I的制备方法包括以下步骤:1)将式I-1所示的化合物(R2为氢的式I化合物)与NBS(N-溴代丁二酰亚胺)或NIS(N-碘代丁二酰亚胺)于二氯甲烷溶液中回流反应,得到式I-2所示化合物;(其中R1为列表中的任意含苯环的芳基基团)2)式I-2所示化合物在间氯过氧苯甲酸作用下开环即可得到式Ⅰ-3所示化合物。其中,步骤1)中式I-1所示的化合物与NBS(N-溴代丁二酰亚胺)或NIS(N-碘代丁二酰亚胺)的摩尔比为1:1.2。步骤2)中式I-2所示的化合物与间氯过氧苯甲酸的摩尔比为1:2.5。当R2为时,所述式I的制备方法包括以下步骤:1)将式I-1所示的化合物与NBS(N-溴代丁二酰亚胺)于二氯甲烷溶液中回流反应,得到式I-2所示的R2为溴的产物;(其中R1为列表中的任意含苯环的芳基基团)2)将式I-2所示的化合物与3-丁炔-1-醇在Pd(dba)2的催化作用下,利用碳酸铯作为添加剂在甲苯中反应,得到式I-3所示的化合物;3)将式I-3所示的化合物在间氯过氧苯甲酸的作用下开环即可得到式I-4所示的化合物。其中,步骤1)中式I-1所示的化合物与NBS的摩尔比为1:1.2。步骤2)中式I-2所示的化合物与Pd(dba)2的摩尔比为1:0.1;式I-2所示的化合物与碳酸铯的摩尔比为1:2;所述反应的反应温度为110℃,反应时间为24小时。步骤3)中式I-2所示的化合物与间氯过氧苯甲酸的摩尔比为1:2.5。当R4为氢、乙基或时,所述式I化合物的制备方法包括下述步骤:1)式Ⅳ所示的化合物与对甲苯磺酰肼在盐酸(0.1equiv)催化下于乙醇(1mmol/5mL)溶液中室温反应1-2h,得到式V化合物;2)式V化合物在等当量碳酸氢钠条件下于二甲基亚砜(1mmol/5mL)溶液中100℃加热发生分子内关环消除反应4h生成式VII化合物(即R4为氢的式I化合物);3)式VII化合物在2倍摩尔量的氢化钠于无水四氢呋喃溶液中与碘乙烷或溴丙炔发生亲核取代反应,得到R4为乙基或炔丙基的式I化合物;4)式I化合物(R4为氢或乙基或炔丙基的化合物)可进行如上所述的进一步氧化开环转化,得到R1为表中所列举各芳基取代基、R2为氢、R3为顺式和反式1,4-二酮、R4为氢、乙基或炔丙基的式I化合物。当R4为时,所述式I的制备方法包括以下步骤:将式I-3所示的化合物与吗啉在4-二甲氨基吡啶、EDCI缩合剂、的三乙胺作用下于无水二氯甲烷溶液中进行反应,得到式I-4所示的化合物。(其中R1为列表中的任意含苯环的芳基基团)上述方法中,所述式I-3所示的化合物、吗啉、4-二甲氨基吡啶、EDCI缩合剂、三乙胺的摩尔比为1:1.2:0.1:1.0:1.0。所述反应的反应为室温,反应时间为12小时。上述制备本发明式I化合物的式Ⅳ所示的化合物及式V所示的化合物以及它们的制备方法也属于本发明的保护范围。本发明的再一个目的是提供式I所示化合物及其药学上可接受的盐的用途。本发明所提供的式I所示化合物及其药学上可接受的盐用途是其在制备抗菌药物中的应用。所述抗菌药物为抗细菌药物。所述细菌为肺炎链球菌、多重耐药的肺炎链球菌(MRSP)、金黄色葡萄球菌、耐甲氧西林金黄色葡萄球菌(MRSA)、粪肠球菌、多重耐药的粪肠球菌(MDEF);耐万古霉素的粪肠球菌(VRE)。本发明中所述的多重耐药菌(multipleresistantbacteria)是指有多重耐药性的病原菌。其定义为一种微生物对三类(比如氨基糖苷类、红霉素、B-内酰胺类)或三类以上抗生素同时耐药。以式I所示的化合物及其药学上可接受的盐为活性成分制备的抗菌药物也属于本发明的保护范围。所述抗菌药物可通过注射、喷射、滴鼻、滴眼、渗透、吸收、物理或化学介导的方法导入机体如肌肉、皮内、皮下、静脉、粘膜组织;或是被其他物质混合或包裹后导入机体。需要的时候,在上述药物中还可以加入一种或多种药学上可接受的载体。所述载体包括 药学领域常规的稀释剂、赋形剂、填充剂、粘合剂、湿润剂、崩解剂、吸收促进剂、表面活性剂、吸附载体、润滑剂等。以式I所示化合物及其药学上可接受的盐为活性成分制备的抗菌药物可以制成注射液、片剂、粉剂、颗粒剂、胶囊、口服液等多种形式。上述各种剂型的药物均可以按照药学领域的常规方法制备。药效学试验证明,本发明的化合物可以有效抑制或杀灭广谱和多重耐药的金黄色葡萄球菌、肺炎链球菌和金黄色葡萄球菌,寄希望于发展一种新型有效并具备新靶点的抗菌药物。附图说明图1为化合物D3的合成路线流程图。图2为化合物D3-1的合成路线流程图。图3为化合物D3-2的合成路线流程图。图4为化合物D3-3的合成路线流程图。图5为化合物D3-4的合成路线流程图。图6为化合物D3-8的合成路线流程图。图7为化合物D3-9的合成路线流程图。图8为化合物D3-10的合成路线流程图。图9为化合物D3-13的合成路线流程图。图10为化合物D3-14的合成路线流程图。图11为化合物D3-15的合成路线流程图。图12为化合物D3-16的合成路线流程图。图13为化合物D3-17的合成路线流程图。图14为化合物D3-18的合成路线流程图。图15为化合物D3-37的合成路线流程图。图16为化合物D3-5的合成路线流程图。图17为化合物D3-6的合成路线流程图。图18为化合物D3-7的合成路线流程图。图19为化合物D3-11的合成路线流程图。图20为化合物D3-12的合成路线流程图。图21为化合物D3-19的合成路线流程图。图22为化合物D3-20的合成路线流程图。图23为化合物D3-21的合成路线流程图。图24为化合物D3-22的合成路线流程图。图25为化合物D3-23的合成路线流程图。图26为化合物D3-25的合成路线流程图。图27为化合物D3-30的合成路线流程图。图28为化合物D3-24的合成路线流程图。图29为化合物D3-31的合成路线流程图。图30为化合物D3-26的合成路线流程图。图31为化合物D3-32和化合物D3-33的合成路线流程图。图32为化合物D3-34的合成路线流程图。图33为化合物D3-35的合成路线流程图。图34为化合物D3-36的合成路线流程图。图35为化合物D3-38和D3-38’的合成路线流程图。图36为化合物D3-39的合成路线流程图。图37为化合物D3-40、D3-41、D3-42的合成路线流程图。图38为化合物D3-43和D3-43’的合成路线流程图。图39为化合物D3-44、D3-45、D3-46的合成路线流程图。图40为化合物D3-48的合成路线流程图。图41为化合物D3-49的合成路线流程图。图42为化合物D3-50的合成路线流程图。图43为化合物D3-51的合成路线流程图。图44为化合物D3-52的合成路线流程图。图45为化合物D3-53和D3-54的合成路线流程图。图46为化合物D3-55的合成路线流程图。图47为化合物D3-56的合成路线流程图。图48为化合物D3-57的合成路线流程图。图49为化合物D3-58的合成路线流程图。图49为化合物D3-58的合成路线流程图。图50为化合物D3-59的合成路线流程图。图51为化合物D3-60的合成路线流程图。图52为化合物D3-61的合成路线流程图。图53为化合物D3-62的合成路线流程图。图54为化合物D3-63的合成路线流程图。图55为化合物D3-64、D3-65的合成路线流程图。图56为化合物D3-67的合成路线流程图。图57为化合物D3-69、D3-70的合成路线流程图。图58为化合物D3-71的合成路线流程图。图59为化合物D3-73、D3-74的合成路线流程图。图60为化合物D3-76的合成路线流程图。图61为化合物D3-77、D3-77’的合成路线流程图。图62为化合物D3-78的合成路线流程图。图63为化合物D3-79的合成路线流程图。图64为化合物D3-80的合成路线流程图。图65为化合物D3-81的合成路线流程图。图66为化合物D3-82的合成路线流程图。图67为化合物D3-83a和化合物D3-83b的合成路线流程图。图68为化合物D3-84的合成路线流程图。图69为化合物D3-85b的合成路线流程图。图70为化合物D3-86的合成路线流程图。图71为化合物D3-87的合成路线流程图。图72为化合物D3-88b的合成路线流程图。图73为化合物D3-89的合成路线流程图。图74为化合物D3-90的合成路线流程图。图75为化合物D3-91、化合物D3-92、化合物D3-93的合成路线流程图。图76为化合物D3-94的合成路线流程图。图77为化合物D3-95的合成路线流程图。图78为化合物D3-96的合成路线流程图。图79为化合物D3-97的合成路线流程图。图80为化合物D3-101的合成路线流程图。图81为化合物D3-102的合成路线流程图。图82为化合物D3-106的合成路线流程图。图83为化合物D3-107的合成路线流程图。图84为化合物D3-108的合成路线流程图。图85为化合物D3-109的合成路线流程图。具体实施方式下面通过具体实施例对本发明的方法进行说明,但本发明并不局限于此。下述实施例中所述实验方法,如无特殊说明,均为常规方法;所述试剂和生物材料,如无特殊说明,均可从商业途径获得。下述实施例中所用的肺炎链球菌、金黄色葡萄球菌、粪肠球菌的菌株具体如下:金黄色葡萄球菌(Staphylococcusaureus):ATCC编号为29213;肺炎链球菌(Streptococcuspneumoniae):NCTC编号为7466;粪肠球菌(Enterococcusfaecalis):ATCC编号为29212实施例中所使用的MRSP:多重耐药的肺炎链球菌;MDEF:多重耐药的粪肠球菌;VRE:耐万古霉素的粪肠球菌;MRSA:耐甲氧西林金黄色葡萄球菌;均为医院的临床分离菌株。其抗药性经过了严格药敏试验验证。公众可从申请人处获得该生物材料,该生物材料只为重复本发明的相关实验所用,不可作为其它用途使用。第一部分、化合物的制备实施例1、制备化合物D31、中间体α,β-不饱和酮a的制备具体操作步骤如下:在圆底烧瓶中加入20.0mL无水乙醇,用冰水浴冷却到0℃,加入1.10mL(10mmol)3-乙酰基吡啶和1.00mL(10mmol)5-甲基糠醛,将480mg氢氧化钠固体溶解于10.0mL乙醇和10.0mL水的混合液中,将氢氧化钠的溶液逐滴加入到底物的溶液中,反应2h。加入饱和氯化铵溶液调节反应液至中性,加入100ml水以进行稀释,然后用二氯甲烷萃取3次(每次加入25ml二氯甲烷),合并三次萃取的有机相,用水洗涤两次后用无水硫酸钠干燥,然后减压旋干溶剂,剩余物用石油醚:乙酸乙酯=6:1-3:1(体积比)过硅胶柱,得到1.6g中间体a(中间体a为浅黄色固体),产率为75%。化合物表征数据如下:1HNMR(400MHz,CDCl3)δ(ppm)9.23(s,1H),8.77(dd,J=1.8Hz,J=5.0Hz,1H),8.28(m,J=1.8Hz,J=8.0Hz,1H),7.56(d,J=14.8Hz,1H),7.42(dd,J=5.0Hz,J=8.0Hz,1H),7.32(d,J=14.8Hz,1H),7.68(d,J=2.8Hz,1H),6.15(d,J=2.8Hz,1H),2.39(s,3H);13CNMR(100MHz,CDCl3)δ(ppm)188.22,156.49,152.83,150.00,149.62,135.60,133.57,131.47,123.47,119.20,116.65,109.62,13.94.2、中间体腙b的制备具体操作步骤如下:在圆底烧瓶中加入α,β-不饱和酮426mg,加入6.0mL无水乙醇溶解,加入醋酸57μL,搅拌10min后,加入苯肼256μL,反应液加热至回流8h,反应液冷却至室温,加入饱和碳酸氢钠溶液和二氯甲烷萃取三次,合并的有机相用饱和食盐水洗。有机相用无水硫酸钠干燥,然后减压旋干溶剂,剩余物用石油醚:乙酸乙酯=4:1-3:1(体积比)过硅胶柱,得到546mg中间体腙b(中间体b为黄色固体),产率为90%。氢谱确结构为腙的顺反混合物,LC-MS分子量确认。3、产物吡唑c的制备具体操作步骤如下:在圆底烧瓶中加入546mg腙b,加入3.0mL无水DMSO溶解,加入对伞花烃二氯化钌二聚体6.1mg,NaHCO3168mg,反应体系用氧气置换保护,加热至110℃搅拌4h。反应液冷却至室温,加入水和二氯甲烷萃取三次,合并的有机相用饱和食盐水洗。有机相用无水硫酸钠干燥,然后减压旋干溶剂,剩余物用二氯甲烷:乙酸乙酯=20:1-15:1(体积比)过硅胶柱,得到188mg吡唑化合物c(白色固体),产率为35%。化合物数据表征如下:1HNMR(400MHz,CDCl3):δ(ppm)9.116(d,J=1.6Hz,1H),8.576(dd,3J=4.8Hz,4J=1.6 Hz,1H),8.208(dt,3J=8.0Hz,4J=1.6Hz,1H),7.507-7.475(m,5H),7.344(dd,3J=8.0Hz,3J=4.8Hz,1H),6.987(s,1H),5.924(d,3J=3.2Hz,1H),5.798(d,3J=3.2Hz,1H),2.310(s,3H);13CNMR(100MHz,CDCl3):δ(ppm)152.83,149.04,148.99,147.32,142.40,140.15,136.59,132.96,129.17,128.88,128.84,126.21,123.56,110.03,107.44,102.37,13.56;LC-MS(ESI+):m/zcalculatedforC19H16N3O(M+H)+:302.13,found:301.22.实施例2-15按照与实施例1基本相同的方法,仅需将实施例1步骤1中3-乙酰基吡啶或5-甲基糠醛及步骤2中的苯肼进行相应的替换即可,其他操作同实施例1,得到下述化合物。4-(5-(5-methylfuran-2-yl)-1-phenyl-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)8.628(d,J=5.12Hz,2H),7.756(d,J=5.12Hz,2H),7.428-7.464(m,5H),7.006(s,1H),5.906(d,J=2.52Hz,1H),5.782(d,J=3.16Hz,1H),2.284(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)152.94,150.27,149.34,142.25,140.34,140.11,136.84,129.21,129.00,126.20,120.14,110.22,107.52,102.96,13.55;LC-MS(ESI+):m/zcalculatedforC19H16N3O(M+H)+:302.13,found302.22.2-(5-(5-methylfuran-2-yl)-1-phenyl-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)8.659(d,J=4.74Hz,1H),8.046(d,J=7.92Hz,1H),7.156(t,J=7.70Hz,1H),7.517-7.435(m,5H),7.284(s,1H),7.231-7.201(m,1H),5.912(d,J=7.08Hz,1H),5.852(d,J=7.08Hz,1H),2.264(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)152.68,152.21,151.90,149.48,142.63,140.37,136.50,136.45,128.99,128.63,126.22,122.67,120.23,109.87,107.29,103.98,13.48;LC-MS(ESI+):m/zcalculatedforC19H16N3O(M+H)+:302.13,found302.21.3-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)9.110(s,1H),8.579(d,J=4.44Hz,1H),8.203(d,J=7.84Hz,1H),7.460-7.432(m,5H),7.345(dd,J=7.80Hz,J=4.88Hz,1H),7.305(d,J=5.08Hz,1H),6.964(t,J=4.24Hz,1H),6.907(s,1H),6.864(d,J=3.60Hz,1H);13C-NMR(100MHz,CDCl3)δ(ppm)149.25,149.11,147.44,139.76,138.75,133.08,130.99,129.26,128.91,128.74,127.63,127.57,126.93,126.33,123.69,105.00;LC-MS(ESI+):m/zcalculatedforC18H14N3S(M+H)+:304.09,found304.19.3-(1-(4-methoxyphenyl)-5-(5-methylfuran-2-yl)-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)9.107(s,1H),8.563(d,J=4.60Hz,1H),8.192(d,J=7.72Hz,1H),7.404(d,J=8.44Hz,2H),7.331(dd,J=7.80Hz,J=4.84Hz,1H),6.997(d,J=8.48Hz,2H),6.966(s,1H),5.911(d,J=2.84Hz,1H),5.733(d,J=3.12Hz,1H),3.877(s,3H),2.317(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)159.93,152.69,148.97,148.70,147.33,142.63,136.88,133.18,132.89,128.97,127.73,123.51,114.32,109.74,107.42,101.71,55.60,13.54;LC-MS(ESI+):m/zcalculatedforC20H18N3O2(M+H)+:332.14,found332.22.3-(5-(furan-2-yl)-1-phenyl-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)9.122(s,1H),8.575(s,1H),8.199(d,J=7.88Hz,1H),7.487-7.451(m,5H),7.422(d,J=1.12Hz,1H),7.338(dd,J=7.76Hz,J=4.84Hz,1H),7.015(s,1H),6.345(dd,J=3.16Hz,J=1.72Hz,1H),5.986(d,J=3.40Hz,1H);13C-NMR(100MHz,CDCl3)δ(ppm)149.10,149.08,147.32,144.08,142.77,140.07,136.14,132.97,129.20,128.82,125.98,123.57,111.32,109.19,103.26;LC-MS(ESI+):m/zcalculatedforC18H14N3O(M+H)+:288.11,found288.20.3-(1-(3-chlorophenyl)-5-(furan-2-yl)-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)9.101(d,J=1.48Hz,1H),8.585(dd,J=4.72Hz,J=1.28Hz,1H),8.187(dt,J=7.92Hz,J=1.84Hz,1H),7.539(t.J=1.64Hz,1H),7.444-7.330(m,5H),6.995(s,1H),6.402(dd,J=3.36Hz,J=1.80Hz,1H),6.162(d,J=3.20Hz,1H);13C-NMR(100MHz,CDCl3)δ(ppm)149.51,149.30,147.32,143.56,143.09,141.02,135.98,134.76,132.99,130.03,128.72,128.49,125.98,123.72,123.59,111.45,109.76,104.06;LC-MS(ESI+):m/zcalculatedforC18H12ClN3O(M+H)+:321.07,found321.52.4-(1-(tert-butyl)-5-(5-methylfuran-2-yl)-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)8.597(d,J=4.48Hz,2H),7.697(d,J=5.12Hz,2H),6.722(s,1H),6.367(d,J=3.00Hz,1H),6.090(d,J=2.40Hz,1H),2.378(s,3H),1.589(s,12H).LC-MS(ESI+):m/zcalculatedforC17H20N3O(M+H)+:282.16,found282.25.4-(4-methyl-5-(5-methylfuran-2-yl)-1-phenyl-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)8.672(d,J=5.36Hz,2H),7.716(d,J=5.48Hz,2H),7.401-7.335(m,5H),6.137(d,J=3.04Hz,1H),6.028(d,J=3.04Hz,1H),2.382(s,3H),2.249(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)153.29,150.15,148.40,141.63,141.41,140.44,133.80,128.93,127.92,124.84,122.15,115.91,112.49,107.31,13.65,10.38;LC-MS(ESI+):m/zcalculatedforC20H18N3O(M+H)+:316.14,found316.16.4-(1-phenyl-5-(1H-pyrrol-2-yl)-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)8.628(d,J=5.40Hz,2H),8.344(bs,1H),7.759(d,J=5.56Hz,2H),7.466-7.436(m,5H),6.847(s,1H),6.780(s,1H),6.200(dd,J=5.56Hz,J=2.72Hz,1H),6.139(s,1H);13C-NMR(100MHz,CDCl3)δ(ppm)150.20,149.48,140.68,140.08,138.28,129.46,128.82,125.78,120.71,120.27,119.76,110.21,109.83,103.30;LC-MS(ESI+):m/zcalculatedforC18H15N4(M+H)+:287.13,found287.22.4-(1-(4-fluorophenyl)-5-(5-methylfuran-2-yl)-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)8.651(d,J=5.24Hz,2H),7.757(d,J=5.40Hz,2H),7.477(dd,J=8.64Hz,J=4.84Hz,2H),7.183(t,J=8.44Hz,2H),7.007(s,1H),5.948(d,J=3.12Hz,1H),5.841(d,J=3.12Hz,1H),2.305(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)162.74(d,J=242.0Hz,C),153.24,150.42,149.62,142.18,140.29,137.05,136.33(d,J=3.6Hz,C),128.21(d,J=8.8Hz,CH),120.22,116.26(d,J=22.9Hz,CH),110.40,107.64,103.15,13.66;LC-MS(ESI+):m/zcalculatedforC19H15FN3O(M+H)+:320.12,found320.11.4-(5-(5-methylfuran-2-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)8.668(d,J=4.96Hz,2H),7.769-7.731(m,4H),7.640(d,J=8.28Hz,2H),7.019(s,1H),6.054(d,J=3.20Hz,1H),6.008(d,J=3.20Hz,1H),2.300(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)153.62,150.49,150.25,143.00,141.66,140.00,136.64,130.60(q,J=33.9Hz,C),126.36(q,J=3.7Hz,CH),125.84,123.89(q,J=269.6Hz,C),120.23,111.18,107.76,104.60,13.66;LC-MS(ESI+):m/zcalculatedforC20H15F3N3O(M+H)+:370.12,found370.15.2-(5-(5-methylfuran-2-yl)-1-phenyl-1H-pyrazol-3-yl)pyrazine1H-NMR(400MHz,CDCl3)δ(ppm)9.305(s,1H),8.594(s,1H),8.498(d,J=2.40Hz,1H),7.516-7.481(m,5H),7.286(s,1H),5.926(d,J=3.20Hz,1H),5.848(d,J=3.20Hz,1H),2.284(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)153.07,149.82,147.73,144.13,143.42,142.42,142.38,140.24,136.87,129.24,129.07,126.32,110.33,107.52,104.38,13.61;LC-MS(ESI+):m/zcalculatedforC18H15N4O(M+H)+:303.12,found303.44.4-(4-methyl-5-(5-methylfuran-2-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)8.689(s,2H),7.713(d,J=5.56Hz,2H),7.643(d,J=8.40Hz,2H),7.512(d,J=8.40Hz,2H),6.306(d,J=3.08Hz,1H),6.100(d,J=2.24Hz,1H),2.351(s, 3H),2.253(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)153.87,150.24,149.22,143.09,141.00,140.77,133.68,129.41(q,J=33.0Hz,C),126.08(q,J=4.0Hz,CH),124.11,124.00(q,J=272.0Hz,C),122.09,117.20,113.23,107.58,13.66,10.32;LC-MS(ESI+):m/zcalculatedforC21H17F3N3O(M+H)+:384.13,found384.48.4-(5-(thiophen-2-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)8.676(dd,J=4.68Hz,J=1.44Hz,2H),7.766(dd,J=4.56Hz,J=1.52Hz,2H),7.692(d,J=8.48Hz,2H),7.585(d,J=8.40Hz,2H),7.393(dd,J=5.08Hz,J=0.84Hz,1H),7.029(dd,J=5.04Hz,J=3.64Hz,1H),6.911(dd,J=3.52Hz,J=0.80Hz,1H);LC-MS(ESI+):m/zcalculatedforC19H13F3N3S(M+H)+:372.08,found372.59.实施例16、制备化合物D3-5实施例16与1-15类似,只是中间体腙的制备方法不同。实施例16中,通过羟醛缩合制备不饱和酮,通过浓硫酸催化脱水缩合生成不饱和腙,通过过渡金属催化的分子内碳氮键偶联生成吡唑产物。化合物数据表征如下:1H-NMR(400MHz,CDCl3)δ(ppm)9.108(s,1H),8.617(d,J=4.44Hz,1H),8.318(d,J=8.80Hz,2H),8.203(d,J=7.92Hz,1H),7.689(d,J=8.84Hz,2H),7.379(dd,J=7.88Hz,J=4.84Hz,1H),6.983(s,1H),6.256(d,J=3.16Hz,1H),6.064(d,J=3.12Hz,1H),2.300(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)153.84,150.40,149.61,147.39,146.60,145.03,141.16,136.21,133.05,128.19,125.04,124.45,123.66,111.72,107.74,105.45,13.58;LC-MS(ESI+):m/zcalculatedforC19H15N4O3(M+H)+:347.11,found347.20.实施例17-18按照与实施例16基本相同的方法,仅需将实施例16步骤2中对硝基苯肼进行相应的替换(如2,4,6-三氯苯肼、盐酸盐的肼)即可,其他操作同实施例15,得到下述化合物。3-(5-(5-methylfuran-2-yl)-1-(2,4,6-trichlorophenyl)-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)9.115(s,1H),8.589(d,J=4.56Hz,1H),8.194(d,J=7.92Hz,1H),7.533(s,2H),7.346(dd,J=7.80Hz,J=4.84Hz,1H),7.041(s,1H),5.946(d,J=2.84Hz,1H),5.727(d,J=3.20Hz,1H),2.290(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)153.27,150.58,149.34,147.46,141.76,137.93,136.57,136.20,134.97,133.18,128.82,128.53,123.55,108.80,107.74,101.09,13.52;LC-MS(ESI+):m/zcalculatedforC19H13Cl3N3O(M+H)+:404.01,found404.08.4-(5-(5-methylfuran-2-yl)-3-(pyridin-4-yl)-1H-pyrazol-1-yl)benzenesulfonamide1H-NMR(400MHz,DMSO-d6)δ(ppm)8.651(s,J=5.28Hz,2H),7.980(d,J=8.36Hz,2H),7.886(d,J=5.56Hz,2H),7.714(d,J=8.40Hz,2H),7.534(s,2H),7.436(s,1H),6.199-6.181(m,2H),2.265(s,3H);LC-MS(ESI+):m/zcalculatedforC19H17N4O3O(M+H)+:381.10,found381.21.实施例19、制备化合物D3-111、中间体α,β-不饱和酮a的制备同实施例1。2、中间体腙b的制备按照与实施例1步骤2基本相同的方法,仅需其中的苯肼替换为对甲苯磺酰肼即可。3、产物吡唑c的制备按照与实施例1步骤3基本相同的方法,仅需去掉其中的钌催化剂即可化合物数据表征如下:1H-NMR(400MHz,CDCl3)δ(ppm)8.591(d,J=5.68Hz,2H),7.659(d,J=5.44Hz,2H),6.782(s,1H),6.505(d,J=3.16Hz,1H),6.013(d,J=2.88Hz,1H),2.293(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)152.58,150.05,120.12,108.06,107.77,99.35,13.64;LC-MS(ESI+):m/zcalculatedforC13H12N3O(M+H)+:226.10,found226.22.实施例20、制备化合物D3-12制备方法如下:在圆底烧瓶中将化合物D3-11溶于无水四氢呋喃溶液中,冰浴冷却,加入氢化钠2.0equiv和碘乙烷2.0equiv,冰浴下搅拌1小时。加入水猝灭反应,后处理后过柱纯化得到两种异构体。经多次纯化后可以得到此主要异构体,产率50%。化合物数据表征如下:4-(1-ethyl-5-(5-methylfuran-2-yl)-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)8.612(d,J=4.24Hz,2H),7.688(d,J=5.88Hz,2H),6.791(s,1H),6.474(d,J=3.20Hz,1H),6.112(d,J=3.20Hz,1H),4.406(q,J=7.24Hz,2H),2.384(s,3H),1.507(t,J=7.24Hz,3H);13C-NMR(100MHz,CDCl3)δ(ppm)153.09,150.32,147.87,142.59,140.82,135.47,120.00,109.96,107.71,102.52,46.49,15.56,13.76;LC-MS(ESI+):m/zcalculatedforC15H16N3O(M+H)+:254.13,found254.18.实施例21、制备化合物D3-19制备方法如下:在圆底烧瓶中将化合物D3-16(37mg,0.1mmol,1.0equiv)溶于2mL二氯甲烷中,加入N-溴丁二酰亚胺(NBS)(21mg,0.12mmol,1.2equiv),反应液加热回流过夜。反应液冷却至室温后,用二氯甲烷和饱和食盐水萃取后处理。合并后的有机相经干燥、过滤、旋干后,粗品经过柱纯化得到白色固体D3-19(29mg,66%产率)。化合物数据表征如下:4-(4-bromo-5-(5-methylfuran-2-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)8.713(d,J=4.72Hz,2H),7.953(d,J=4.88Hz,2H),7.680(d,J=8.20Hz,2H),7.516(d,J=8.20Hz,2H),6.706(d,J=1.76Hz,1H),6.141(d,J=1.76Hz,1H),2.201(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)154.42,150.26,147.79,142.94,139.15,139.03,134.75,130.26(q,J=32.0Hz,C),126.16(q,J=4.0Hz,CH),124.50,123.87(q,J=270.0Hz,C),121.99,114.69,107.87,95.89,13.59;LC-MS(ESI+):m/zcalculatedforC20H14BrF3N3O(M+H)+:448.03,found448.20.实施例22、制备化合物D3-20制备方法如下:圆底烧瓶中将化合物D3-19(26mg,0.058mmol,1.0equiv)与对三氟甲氧基苯硼酸(18mg,0.087mmol,1.5equiv)溶于4mL混合溶剂中(甲苯:乙醇:水/20:5:1),加入催化剂四三苯基磷钯(6.7mg,0.1equiv),三苯基磷(3.0mg,0.2equiv),碳酸钾(20mg,2.5equiv),反应混合液于80℃油浴中加热搅拌过夜。冷却至室温后,用二氯甲烷和饱和食盐水做萃取后处理。经过柱纯化(石油醚:乙酸乙酯/5:1-3:1-1:1)得到产物D3-20与原料D3-19的混合物17mg,比例为3:1。化合物数据表征如下:4-(5-(5-methylfuran-2-yl)-4-(4-(trifluoromethoxy)phenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)pyridine(D3-20)1H-NMR(400MHz,CDCl3)δ(ppm)8.540(d,J=4.56Hz,2H),7.694(d,J=8.40Hz,2H),7.578(d,J=8.32Hz,2H),7.402(d,J=5.56Hz,2H),7.301(d,J=8.56Hz,2H),7.221(d,J=8.28Hz,2H),6.037(d,J=3.04Hz,1H),5.954(d,J=3.04Hz,1H),2.155(s,3H),1.255(s,3H);LC-MS(ESI+):m/zcalculatedforC27H18F6N3O2(M+H)+:530.13,found530.30.实施例23、制备化合物D3-211、中间体α,β-不饱和酮a的制备参照实施例1步骤1的方法进行2、中间体腙b的制备:参照实施例1步骤2的方法进行。3、产物吡唑c的制备:参照实施例1步骤3的方法进行。化合物数据表征如下:4-(5-(furan-2-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)pyridine1H-NMR(400MHz,CDCl3)δ(ppm)8.668(d,J=5.36Hz,2H),7.761(d,J=6.00Hz,2H),7.732(d,J=8.48Hz,2H),7.605(d,J=8.32Hz,2H),7.451(d,J=1.12Hz,1H),7.049(s,1H),6.432(dd,J=3.36Hz,J=1.80Hz,1H),6.245(d,J=3.28Hz,1H);13C-NMR(100MHz,CDCl3)δ(ppm)150.42,150.26,143.46,143.33,142.85,139.89,136.13, 130.55(q,J=33.0Hz,C),126.40(q,J=4.0Hz,CH),125.55,123.84(q,J=271.0Hz,C),120.20,111.63,110.33,105.41;LC-MS(ESI+):m/zcalculatedforC19H13F3N3O(M+H)+:356.10,found356.30.实施例24、制备化合物D3-221、中间体α,β-不饱和酮a的制备:参照实施例1步骤1的方法进行。2、于圆底烧瓶中将底物不饱和酮(426mg,2.0mmol,1.0equiv)和对三氟甲基苯肼(423mg,1.2equiv)溶于8.0mL甲醇中,加入几滴浓硫酸,加热回流6h,反应冷却至室温后,旋干溶剂后用二氯甲烷和饱和碳酸氢钠溶液做萃取后处理。合并后的有机相经过干燥、过滤、旋干后过柱纯化(二氯甲烷:乙酸乙酯/15:1-5:1-3:1)得到黄色固体D3-22,产率为60%。实施例25、制备化合物D3-231、中间体α,β-不饱和酮a的制备:参照实施例1步骤1的方法进行。2、方法同实施例24。实施例26、制备化合物D3-25制备方法如下:将化合物D3-16(185mg,0.5mmol,1.0equiv)溶于5.0mL二氯甲烷溶液中,加入间氯过氧苯甲酸m-CPBA(91mg,0.525mmol,1.05equiv),反应于室温下搅拌1小时。用二氯甲烷和饱和碳酸氢钠溶液萃取后处理,合并的有机相经过干燥、过滤、旋干后在硅胶柱上纯化(二氯甲烷:甲醇/40:1-20:1-15:1)得到白色固体D3-25,产率为90%。两者条件相同,但所用间氯过氧苯甲酸的当量不一样,所以产物不一样,D3-25是D3-26的原料化合物数据表征如下:4-(5-(5-methylfuran-2-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)pyridine1-oxide1H-NMR(400MHz,CDCl3)δ(ppm)8.238(d,J=7.00Hz,2H),7.765-7.722(m,4H),7.608(d,J=8.32Hz,2H),6.930(s,1H),6.041(d,J=3.20Hz,1H),5.997(d,J=2.80Hz,1H),2.285(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)153.77,148.57,142.81,141.36,139.51,136.98,130.91(q,J=6.10Hz,CH),126.37(q,J=3.80Hz,CH),125.77,122.59,111.38,107.80,104.10,13.63;LC-MS(ESI+):m/zcalculatedforC20H15F3N3O2(M+H)+:386.11,found386.24.实施例27、制备化合物D3-30制备方法如下:将化合物D3-21(43mg,0.12mmol,1.0equiv)溶于2.0mL无水N,N-二甲基甲酰胺中,冰浴冷却,加入三氯氧磷(112μl,10.0equiv),反应在室温下搅拌1h。反应液用水和二氯甲烷萃取出来,合并后的有机相经过干燥、过滤、旋干后得到的粗品经过柱纯化(石油醚:乙酸乙酯/1:1-1:2)后得到白色固体D3-30(4mg,产率9%)。化合物数据表征如下:5-(3-(pyridin-4-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)furan-2-carbaldehyde1H-NMR(400MHz,CDCl3)δ(ppm)9.650(s,1H),8.701(d,J=4.32Hz,2H),7.817-7.766(m,4H),7.660(d,J=8.20Hz,2H),7.324(s,1H),7.210(d,J=3.60Hz,1H),6.228(d,J=3.56Hz,1H);LC-MS(ESI+):m/zcalculatedforC20H13F3N3O2(M+H)+:384.10,found384.34.实施例28、制备化合物D3-24制备方法如下:圆底烧瓶中加入底物D3-16(181mg,0.49mmol,1.0equiv),氧化剂过硫酸胺(168mg,0.735mmol,1.5equiv),AcOH(0.3mL),DCE(2.5mL),水(2.5mL),反应混合液于50℃油浴中加热搅拌过夜。反应液冷却至室温后,加入二氯甲烷稀释,加入饱和碳酸氢钠,用二氯甲烷萃取水相直至产品全部萃出。合并有机相,干燥、过滤、旋干后的粗品经过硅胶柱纯化(二氯甲烷:乙酸乙酯/2:1-1:1,然后二氯甲烷:丙酮/2:1)得到浅黄色固体D3-24 (95mg,50%产率)。化合物数据表征如下:(E)-1-(3-(pyridin-4-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)8.714(d,J=5.92Hz,2H),7.785-7.759(m,4H),7.597(d,J=8.28Hz,2H),7.511(s,1H),7.494(d,J=15.6Hz,1H),7.138(d,J=15.6Hz,1H),2.428(s,3H);LC-MS(ESI+):m/zcalculatedforC20H15F3N3O2(M+H)+:386.11,found386.29.实施例29、制备化合物D3-31制备方法基本同化合物D3-24,只需将底物由化合物D3-16替换为化合物D3-21。化合物数据表征如下:(E)-4-oxo-4-(3-(pyridin-4-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)but-2-enal1H-NMR(400MHz,CDCl3)δ(ppm)9.908(d,J=7.00Hz,1H),8.719(d,J=5.68Hz,2H),7.797-7.764(m,4H),7.609(d,J=8.28Hz,2H),7.528(d,J=14.84Hz,1H),7.509(s,1H),7.056(dd,J=15.72Hz,J=7.04Hz,1H),LC-MS(ESI+):m/zcalculatedforC19H35F3N3O2(M+H)+:372.10,found372.29.实施例30、制备化合物D3-26制备方法如下:圆底烧瓶中将底物D3-16(42mg,0.114mmol,1.0equiv)溶解于1mL二氯甲烷(DCM)中,加入间氯过氧苯甲酸(m-CPBA)(78.5mg,0.455mmol,4.0equiv),室温下搅拌5h,加入二氯甲烷和饱和碳酸氢钠溶液做萃取,萃取后的有机相经过干燥、过滤、旋干后经过硅胶柱纯化(二氯甲烷:甲醇/30:1-15:1)得到淡黄色固体产物D3-26(15mg,34%产率)。化合物数据表征如下:(Z)-4-(5-(4-oxopent-2-enoyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)pyridine1-oxide1H-NMR(400MHz,CDCl3)δ(ppm)8.233(d,J=7.12Hz,2H),7.826(d,J=8.44Hz,2H), 7.764(d,J=8.52Hz,2H),7.724(d,J=7.16Hz,2H),7.130(s,1H),6.690(d,J=11.68Hz,1H),6.646(d,J=11.68Hz,1H),2.315(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)198.28,182.99,148.00,142.73,140.96,139.66,136.31,135.86,129.89,126.19(q,J=3.6Hz,CH),125.81,122.65,30.21;LC-MS(ESI+):m/zcalculatedforC20H15F3N3O3(M+H)+:402.11,found402.28.实施例31、制备化合物D3-32和化合物D3-33制备方法如下:将化合物D3-16(36.9mg,0.1mmol,1.0equiv)溶解于1.2mL叔丁醇与水的混合液中(5:1),加入亚氯酸钠(54mg,0.6mmol,6.0equiv),水合磷酸二氢钠(42mg,0.3mmol,3.0equiv)反应混合液于室温下搅拌4h。用乙酸乙酯与饱和碳酸氢钠溶液后处理(一般萃取),经干燥、过滤、旋干后的粗品硅胶柱纯化(二氯甲烷:丙酮/3:1-2:1)得到分离后的两种化合物D3-32(主产物,10mg)和D3-33(少量产物,3mg)化合物数据表征如下:1-(3-(3-(pyridin-4-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazole-5-carbonyl)oxiran-2-yl)ethanone1H-NMR(400MHz,CDCl3)δ(ppm)8.704(d,J=5.80Hz,2H),7.775-7.743(m,4H),7.649(s,1H),7.565(d,J=8.36Hz,2H),4.095(d,J=1.64Hz,1H),3.732(d,J=1.64Hz,1H),2.208(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)201.90,182.38,150.75,150.35,142.66,138.57,138.07,126.34,126.29,120.15,58.86,56.59,25.09;LC-MS(ESI+):m/zcalculatedforC20H15F3N3O3(M+H)+:402.11,found402.28.1-(3-(3-(pyridin-4-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazole-5-carbonyl)oxiran-2-yl)ethanone1H-NMR(400MHz,CDCl3)δ(ppm)8.710(d,J=5.08Hz,2H),7.778-7.746(m,4H),7.666(s,1H),7.596(d,J=8.40Hz,2H),4.192(d,J=4.80Hz,1H),3.882(d,J=4.80Hz,1H),2.331(s,3H);LC-MS(ESI+):m/zcalculatedforC20H15F3N3O3(M+H)+:402.11,found402.33.实施例32、制备化合物D3-34制备方法如下:将化合物D3-24(19mg,0.05mmol,1.0equiv)溶解于1mL甲醇中,加入硼氢化钠(2-3mg,1.2equiv),反应液于冰浴条件下搅拌20min。加入水猝灭反应,用乙酸乙酯和弱酸溶液后处理后,干燥、过滤、旋干后的粗品经过硅胶柱纯化(二氯甲烷:甲醇/20:1-10:1)得到以上混合物D3-34(核磁数据:比例约为0.55:0.45)。LC-MS(ESI+):m/zcalculatedforC20H21F3N3O2(M+H)+:392.16,found392.33.LC-MS(ESI+):m/zcalculatedforC20H19F3N3O2(M+H)+:390.14,found390.33.实施例33、制备化合物D3-35制备方法如下:将化合物D3-24(19mg,0.05mmol,1.0equiv)溶解于2mL甲醇溶液中,加入10%Pd/C(10mg),反应瓶密封后用氢气球交换三次。反应液于室温下剧烈搅拌5h。硅藻土过滤掉钯碳,旋干溶剂甲醇,残留物经过硅胶柱纯化(石油醚:丙酮/3:1-1:1)得到白色固体D3-35(16mg,80%产率)。1-(3-(pyridin-4-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pentane-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)8.689(d,J=5.20Hz,2H),7.753-7.722(m,4H),7.575(d,J=8.28Hz,2H),7.460(s,1H),3.208(t,J=6.00Hz,2H),2.881(t,J=6.00Hz,2H),2.208(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)206.60,188.86,150.65,149.72,140.14(q,J=204Hz,C),130.90(q,J=33Hz,C),136.31,126.08(q,J=3.0Hz,CH),120.16,110.36,37.00,34.42,29.85;LC-MS(ESI+):m/zcalculatedforC20H17F3N3O2(M+H)+:388.13,found388.33.实施例34、制备化合物D3-36制备方法如下:将化合物D3-31(17mg,0.046mmol,1.0equiv)溶解于0.6mL四氢呋喃和0.6mL叔丁醇中。将氨基磺酸(36mg,0.37mmol,8.0equiv),亚氯酸钠(13mg,0.137mmol,3.0equiv),一水合磷酸二氢钠(45mg,0.322mmol,7.0equiv)溶解于0.6mL水中。将上述溶液滴加到底物的溶液中。反应液在室温下搅拌过夜。后处理用乙酸乙酯和水,干燥、过滤、旋干后的粗品经过硅胶柱纯化(二氯甲烷:甲醇/20:1-7:1-5:1)得到5mg的白色固体D3-36。(E)-4-oxo-4-(3-(pyridin-4-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)but-2-enoicacid1H-NMR(400MHz,CDCl3)δ(ppm)8.672(d,J=5.44Hz,2H),7.733-7.718(m,4H),7.578(d,J=8.24Hz,2H),7.469(s,1H),7.406(d,J=15.56Hz,1H),7.050(d,J=15.56Hz,1H)。实施例35、制备化合物D3-38和D3-38’制备方法参见图35。后处理后的粗品经过硅胶柱纯化(二氯甲烷:丙酮/2:1-1:1)得到以上两种的混合物,两者的比例经过核磁确认。(Z)-4-(5-(4-oxopent-2-enoyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)-2-(4-(trifluoromethoxy)phenyl)pyridine1-oxide1H-NMR(400MHz,CDCl3)δ(ppm)8.417-8.383(m,1.65H),7.627(s,1H),7.159(s,0.66H),6.688(d,J=11.60Hz,0.65H),6.645(d,J=11.60Hz,0.65H),4.146(d,J=4.40Hz,1H),3.889(d,J=4.40Hz,1H),2.332(s,3H),2.315(s,1.98H);LC-MS(ESI+):m/zcalculatedforC27H18F6N3O4(M+H)+:562.12,found562.33.LC-MS(ESI+):m/zcalculatedforC27H18F6N3O5(M+H)+:578.12,found578.32.实施例36、制备化合物D3-39制备方法如下:将化合物D3-24(32mg,0.083mmol,1.0equiv)溶解于2.0mL二氯甲烷中,加入间氯过氧苯甲酸(36mg,0.208mmol,2.5equiv),反应液于室温下搅拌3h。用二氯甲烷和饱和碳酸氢钠溶液正常后处理,干燥、过滤、旋干后的粗品经过硅胶柱的纯化(洗脱体系为二氯甲烷:甲醇/30:1-25:1)得到24mg浅黄色固体产物D3-39,产率为73%。(E)-4-(5-(4-oxopent-2-enoyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)pyridine1-oxide1H-NMR(400MHz,CDCl3)δ(ppm)8.314(d,J=6.56Hz,2H),7.802-7.769(m,4H),7.577(d,J=8.24Hz,2H),7.465(d,J=15.56Hz,1H),7.430(s,1H),7.137(d,J=15.60Hz,1H),2.424(s,3H);LC-MS(ESI+):m/zcalculatedforC20H15F3N3O3(M+H)+:402.11,found402.03.实施例37、制备化合物D3-40、D3-41、D3-42制备方法参见图37。其中D3-40和D3-41为同一反应生成的两种产物。D3-42为相同底物不同条件生成的产物。对于D3-40和D3-41,反应后粗品经过硅胶柱纯化(第一次:石油醚:乙酸乙酯/3:1-2:1;第二次:石油醚:二氯甲烷/2:1-1:1-1:2)对于D3-42,方法同D3-43。硅胶柱纯化体系酮D3-40.(Z)-1-(3-phenyl-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione(化合物D3-40)1H-NMR(400MHz,CDCl3)δ(ppm)7.862-7.830(m,4H),7.751(d,J=8.28Hz,2H),7.441(t,J=7.28Hz,2H),7.378(t,J=7.04Hz,1H),7.167(s,1H),6.715(d,J=11.76Hz,1H),6.609(d,J=11.76Hz,1H),2.302(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)198.76,182.78,152.33,143.22,140.35,136.56,135.65,131.68,130.36(q,J=33.0Hz,C),128.96,128.93,126.04(q,J=4.0Hz,CH),125.95,125.86,110.48,30.08;(E)-1-(3-phenyl-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione(化合物D3-41)1H-NMR(400MHz,CDCl3)δ(ppm)7.892(d,J=7.64Hz,2H),7.758(d,J=8.24Hz,2H),7.608(d,J=8.20Hz,2H),7.505(d,J=15.44Hz,1H),7.468-7.387(m,4H),7.129(d,J=15.44Hz,1H),2.425(s,3H);LC-MS(ESI+):m/zcalculatedforC21H16F3N2O2(M+H)+:385.12,found385.31.1-(3-(3-phenyl-1-(4-(trifluoromethyl)phenyl)-1H-pyrazole-5-carbonyl)oxiran-2-yl)ethanone(化合物D3-42)1H-NMR(400MHz,CDCl3)δ(ppm)7.886(d,J=7.60Hz,2H),7.734(d,J=8.28Hz,2H),7.590(d,J=8.28Hz,2H),7.552(s,1H),7.458(t.J=7.40Hz,2H),7.397(t,J=7.24Hz,1H),4.221(d,J=4.84Hz,1H),3.834(d,J=4.84Hz,1H),2.302(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)202.59,182.25,152.85,142.86,138.88,131.38,130.76(q,J=33.0Hz,C),129.13,129.03,126.16,126.13,126.06,123.91(q,J=271.0Hz,C),111.61,59.18,57.81,28.64;LC-MS(ESI+):m/zcalculatedforC21H16F3N2O3(M+H)+:401.11,found401.01.实施例38、制备化合物D3-43和D3-43’制备方法参见图38。圆底烧瓶中加入吡唑(156mg,0.45mmol,1.0equiv),亚氯酸钠(123mg,1.35mmol,3.0equiv),水合磷酸二氢钠(311mg,2.25mmol,5.0equiv),2-甲基丁二烯(756μL,9.0mmol,20.0equiv),四氢呋喃2mL,叔丁醇3mL和水1mL,反应液与室温下搅拌12h。用二氯甲烷和水后处理,干燥、过滤、旋干后粗品经过硅胶柱纯化(梯度洗脱体系为二氯甲烷:乙酸乙酯/2:1-1:1-1:2-1:3)得到混合物D3-43和D3-43’(比例通过核磁确认)。产物为白色固体,转化率较低,大部分原料剩余。1H-NMR(400MHz,CDCl3)δ(ppm)8.725(s,2H),8.363(d,J=8.24Hz,2H),7.781-7.753(m,2H),7.700-7.622(m,3H),4.189(d,J=4.68Hz,0.60H),4.113(d,J=1.64Hz,0.40H),3.914(d,J=4.68Hz,0.60H),3.749(d,J=1.64Hz,0.40H)LC-MS(ESI+):m/zcalculatedforC19H15N4O5(M+H)+:379.10,found379.36.实施例39、制备化合物D3-44、D3-45、D3-46制备方法参见图39。反应后的粗品经过硅胶柱纯化(第一次纯化:石油醚:乙酸乙酯/10:1-5:1-3:1),对于相对较纯的D3-45和D3-46,再次硅胶柱纯化(石油醚:二氯甲烷/1:1-二氯甲烷:丙酮/20:1-10:1)得到纯品的D3-45(浅黄色固体)和D3-46(浅黄色固体)。methyl4-(3-phenyl-1H-pyrazol-1-yl)benzoate(化合物D3-44)1H-NMR(400MHz,CDCl3)δ(ppm)8.126(d,J=8.56Hz,2H),7.982(d,J=2.24Hz,1H),7.927(d,J=7.48Hz,2H),7.834(d,J=8.56Hz,2H),7.446(t,J=7.44Hz,2H),7.363(t,J=7.24Hz,1H),6.786(d,J=2.20Hz,1H),3.928(s,3H);(E)-methyl4-(5-(4-oxopent-2-enoyl)-3-phenyl-1H-pyrazol-1-yl)benzoate(化合物D3-45)1H-NMR(400MHz,CDCl3)δ(ppm)8.162(d,J=8.28Hz,2H),7.890(d,J=7.52Hz,2H),7.546(d,J=8.40Hz,2H),7.509-7.377(m,5H),7.105(d,J=15.68Hz,1H),3.956(s,3H),2.406(s,3H);LC-MS(ESI+):m/zcalculatedforC22H19N2O4(M+H)+:375.13,found375.03.(Z)-methyl4-(5-(4-oxopent-2-enoyl)-3-phenyl-1H-pyrazol-1-yl)benzoate(化合物D3-46)1H-NMR(400MHz,CDCl3)δ(ppm)8.157(d,J=8.36Hz,2H),7.847(d,J=7.60Hz,2H),7.752(d,J=8.36Hz,2H),7.428(t,J=7.04Hz,2H),7.365(t,J=7.04Hz,1H),7.169(s,1H),6.700(d,J=11.80Hz,1H),6.583(d,J=11.80Hz,1H),3.944(s,3H),2.293(s,3H)。实施例40、制备化合物D3-48制备方法参见图40。具体方法如下:在25mL圆底烧瓶中将不饱和酮(545mg,2.0mmol,1.0equiv),对三氟甲基苯肼(388mg,2.2mmol,1.1equiv)混合溶于5.0mL乙醇中,滴加入0.2mL浓硫酸,加热反应回流24h,将反应液冷却至室温,将溶剂旋干,用二氯甲烷和饱和碳酸氢钠萃取后处理。干燥、过滤、旋干后,粗品经过硅胶柱纯化(石油醚:乙酸乙酯/20:1-10:1)得到较纯消除产物D3-48,含有的少量未消除的吡唑啉中间体,通过氧化反应生成5-取代的吡唑,可经过硅胶出简单纯化得到纯品D3-48(白色固体,80%产率)。3-(3,4-dimethoxyphenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazole1H-NMR(400MHz,CDCl3)δ(ppm)7.816(s,1H),7.764(d,J=8.32Hz,2H),7.599(d,J=8.32Hz,2H),7.491(s,1H),7.342(d,J=8.24Hz,1H),6.848(d,J=8.28Hz,1H),6.618(s,1H),3.928(s,3H),3.844(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)153.38,149.41,149.18,142.32,127.80,127.48(q,J=33.0Hz,C),126.41(q,J=4.0Hz,CH),125.88,123.98(q,J=270.0Hz,CF3),118.60,118.13,111.15,108.85,105.43,55.77,55.69;LC-MS(ESI+):m/zcalculatedforC18H16F3N2O2(M+H)+:349.12,found349.33.实施例41、制备化合物D3-49制备方法参见图41。(Z)-1-(3-(3,4-dimethoxyphenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.814(d,J=8.20Hz,2H),7.720(d,J=8.24Hz,2H),7.423(s,1H),7.323(d,J=8.24Hz,1H),7.094(s,1H),6.894(d,J=8.20Hz,1H),6.681(d,J=11.76Hz,1H),6.564(d,J=11.76Hz,1H),3.926(s,3H),3.885(s,3H),2.267(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)198.82,182.66,152.13,149.80,149.39,143.14,140.21,136.56,135.42,130.09(q,J=33.0Hz,C),125.89(q,J=4.0Hz,CH),125.79,124.56,123.96(q, J=270.0Hz,CF3),118.64,111.33,110.10,108.93,56.03,55.95,29.94;LC-MS(ESI+):m/zcalculatedforC23H20F3N2O4(M+H)+:445.14,found445.37.实施例42、制备化合物D3-50制备方法参见图42。3-(3,4-difluorophenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazole1H-NMR(400MHz,CDCl3)δ(ppm)7.941(d,J=2.52Hz,1H),7.831(d,J=8.44Hz,2H),7.755-7.687(m,1H),7.698(d,J=8.44Hz,2H),7.597-7.566(m,1H),7.229-7.162(m,1H),6.701(d,J=2.52Hz,1H);LC-MS(ESI+):m/zcalculatedforC16H10F5N2(M+H)+:325.08,found325.30.实施例43、制备化合物D3-51制备方法参见图43。(Z)-1-(3-(3,4-difluorophenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.823(d,J=8.36Hz,2H),7.746(d,J=8.44Hz,2H),7.687-7.640(m,1H),7.564-7.538(m,1H),7.237-7.170(m,1H),7.092(s,1H),6.696(d,J=11.72Hz,1H),6.624(d,J=11.72Hz,1H),2.304(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)198.67,182.88,152.08,151.97,151.92,150.34,149.58,149.41,142.98,140.56,136.30,135.84,130.68,130.35,128.94,128.90,128.88,128.84,126.13,126.09,126.05,126.02,125.82,125.32,122.61,122.11,122.07,122.05,122.01,117.91,117.74,115.80,114.90,110.09,30.08;LC-MS(ESI+):m/zcalculatedforC21H14F5N2O2(M+H)+:421.10,found420.99.实施例44、制备化合物D3-52制备方法参见图44。methyl4-(1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)benzoate1H-NMR(400MHz,CDCl3)δ(ppm)8.103(d,J=8.16Hz,2H),8.002(d,J=2.08Hz,1H),7.972(d,J=8.16Hz,2H),7.881(d,J=8.40Hz,2H),7.716(d,J=8.40Hz,2H),6.846(d,J=2.08Hz,1H),3.934(s,3H);LC-MS(ESI+):m/zcalculatedforC18H14F3N2O2(M+H)+:347.10,found347.33.实施例45、制备化合物D3-53和D3-54制备方法参见图45。粗品经过硅胶柱纯化(洗脱体系石油醚:乙酸乙酯/15:1-7:1-5:1-3:1-1:1,然后石油醚:二氯甲烷/1:2-1:9)得到30%产率的D3-53和30%产率的D3-54。化合物D3-53的结构确证数据:(E)-methyl4-(5-(4-oxopent-2-enoyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)benzoate1H-NMR(400MHz,CDCl3)δ(ppm)8.119(d,J=8.24Hz,2H),7.955(d,J=8.20Hz,2H),7.756(d,J=8.32Hz,2H),7.598(d,J=8.24Hz,2H),7.490(d,J=15.60Hz,1H),7.477(s,1H),7.123(d,J=15.60Hz,1H),3.935(s,3H),2.418(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)197.24,179.19,166.78,151.54,142.97,141.01,139.12,135.62,134.24,130.37(q,J=33.0Hz,C),130.38,126.25,126.18(q,J=4.0Hz,CH),125.82,111.36,52.34,29.44;LC-MS(ESI+):m/zcalculatedforC23H18F3N2O4(M+H)+:443.12,found443.37.化合物D3-54的结构确证数据:(Z)-methyl4-(5-(4-oxopent-2-enoyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)benzoate1H-NMR(400MHz,CDCl3)δ(ppm)8.075(d,J=8.20Hz,2H),7.896(d,J=8.16Hz,2H),7.830(d,J=8.32Hz,2H),7.735(d,J=8.32Hz,2H),6.686(d,J=11.76Hz,1H),6.598(d,J=11.76Hz,1H),3.901(s,3H),2.276(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)198.63,182.81,166.70,151.04,143.01,140.49,136.33,135.84,135.71,130.39(q,J=33.0Hz,C),130.21,130.17,125.99(q,J=2.0Hz,CH),125.79,125.66,123.92(q,J=270.0Hz,CF3)110.69,52.18,29.99;LC-MS(ESI+):m/zcalculatedforC23H18F3N2O4(M+H)+:443.12,found443.32.实施例46、制备化合物D3-55制备方法参见图46。(Z)-N-cyclopropyl-4-(5-(4-oxopent-2-enoyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)benzamide1H-NMR(400MHz,CDCl3)δ(ppm)7.856-7.716(m,8H),7.166(s,1H),6.694(d,J=11.76Hz,1H),6.608(d,J=11.76Hz,1H),6.546(s,1H),2.878(s,1H),2.287(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)198.71,182.90,168.46,151.15,143.00,140.46,136.33,135.84,134.55,134.46,130.50(q,J=33.0Hz,C),127.59,126.07(q,J=4.0Hz,CH),125.86,125.82,123.93(q,J=271.0Hz,CF3),110.61,30.13,23.31,6.80.LC-MS(ESI+):m/zcalculatedforC25H21F3N3O3(M+H)+:468.15,found468.38.实施例47、制备化合物D3-56制备方法参见图47。(Z)-N-(4-(5-(4-oxopent-2-enoyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)phenyl)acetamide1H-NMR(400MHz,CDCl3)δ(ppm)7.823-7.767(m,4H),7.734(d,J=8.52Hz,2H),7.574(d,J=8.52Hz,2H),7.436(s,1H),7.110(s,1H),6.717(d,J=11.76Hz,1H),6.619(d,J=11.76Hz,1H),2.308(s,3H),2.183(s,3H)实施例48、制备化合物D3-57制备方法参见图48。(Z)-4-(5-(4-oxopent-2-enoyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)benzoicacid1H-NMR(400MHz,CDCl3)δ(ppm)8.168(d,J=8.40Hz,2H),7.958(d,J=8.48Hz,2H),7.854(d,J=8.44Hz,2H),7.769(d,J=8.40Hz,2H),7.229(s,1H),6.732(d,J=11.76Hz,1H),6.651(d,J=11.76Hz,1H),2.234(s,3H);LC-MS(ESI+):m/zcalculatedforC22H16F3N2O4(M+H)+:429.11,found429.38.实施例49、制备化合物D3-58制备方法参见图49。(Z)-1-(3-methyl-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.987(d,J=7.72Hz,2H),7.583(d,J=7.84Hz,2H),6.682(s,1H),6.654(d,J=11.80Hz,1H),6.566(d,J=11.80Hz,1H),2.358(s,3H),2.299(s,3H);LC-MS(ESI+):m/zcalculatedforC16H14F3N2O2(M+H)+:323.10,found323.35.实施例50、制备化合物D3-59制备方法参见图50。(Z)-4-(5-(4-oxopent-2-enoyl)-3-phenyl-1H-pyrazol-1-yl)benzoicacid1H-NMR(400MHz,CDCl3)δ(ppm)8.252(d,J=8.24Hz,2H),7.865(d,J=8.40Hz,2H),7.821 (d,J=8.60Hz,2H),7.443(t,J=7.28Hz,2H),7.379(t,J=7.28Hz,1H),7.186(s,1H),6.730(d,J=11.76Hz,1H),6.619(d,J=11.76Hz,1H),2.323(s,3H);LC-MS(ESI+):m/zcalculatedforC21H17N2O4(M+H)+:361.12,found361.37.实施例51、制备化合物D3-60制备方法参见图51。(Z)-1-(3-(4-hydroxyphenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.764(d,J=8.44Hz,2H),7.686(d,J=8.56Hz,2H),7.604(d,J=8.64Hz,2H),7.021(s,1H),6.754(d,J=8.64Hz,2H),6.685(d,J=11.76Hz,1H),6.582(d,J=11.76Hz,1H),2.298(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)200.36,182.78,156.84,152.33,143.02,140.00,136.99,135.15,130.23(q,J=33.0Hz,C),127.39,125.99(q,J=4.0Hz,CH),125.90,123.95(q,J=270.0Hz,CF3),115.89,110.27,30.02;LC-MS(ESI+):m/zcalculatedforC21H16F3N2O3(M+H)+:401.11,found401.35.实施例52、制备化合物D3-61制备方法参见图52。(Z)-1-(1-(4-nitrophenyl)-3-phenyl-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)8.324(d,J=8.84Hz,2H),7.930(d,J=8.88Hz,2H),7.843(d,J=7.32Hz,2H),7.459-7.366(m,3H),7.180(s,1H),6.735(d,J=11.68Hz,1H),6.663(d,J=11.68Hz,1H),2.313(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)198.57,183.24,152.76,146.99,145.18,140.51,136.26,136.04,131.32,129.12,129.03,128.98,125.94,125.93,124.27,111.05,30.14;LC-MS(ESI+):m/zcalculatedforC20H16N3O4(M+H)+:362.11,found362.02.实施例53、制备化合物D3-62制备方法参见图53。(Z)-1-(3-(3,4-difluorophenyl)-1-(4-methoxyphenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.695-7.642(m,1H),7.576-7.526(m,1H),7.504(d,J=8.80Hz,2H),7.224-7.158(m,1H),6.982(d,J=8.80Hz,2H),6.626(d,J=11.84Hz,1H),6.507(d,J=11.84Hz,1H),3.857(s,3H),2.283(s,3H);LC-MS(ESI+):m/zcalculatedforC21H17F2N2O3(M+H)+:383.12,found383.02.实施例54、制备化合物D3-63制备方法参见图54。(Z)-1-(3-(3,4-difluorophenyl)-1-(4-hydroxyphenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.668-7.648(m,1H),7.588-7.527(m,1H),7.390-7.368(m,1H),7.234(d,J=8.68Hz,2H),7.091(s,1H),6.823(d,J=8.84Hz,2H),6.650(d,J=11.76Hz,1H),6.540(d,J=11.76Hz,1H),2.182(s,3H);LC-MS(ESI+):m/zcalculatedforC20H15F2N2O3(M+H)+:369.11,found369.32.实施例55、制备化合物D3-64和化合物D3-65制备方法参见图55。粗品经过硅胶柱纯化(洗脱体系石油醚:乙酸乙酯/4:1-2:1)得到少量D3-64和主产物D3-65(仍含有<10%量的D3-64(核磁数据))化合物D3-64的结构确证数据:(E)-1-(3-(3,4-difluorophenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)hex-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.758(d,J=8.36Hz,2H),7.730-7.710(m,1H),7.594-7.574(m,3H),7.534(d,J=15.52Hz,1H),7.376(s,1H),7.280-7.234(m,1H),7.167(d,J=15.52Hz,1H),2.712(q,J=7.20Hz,2H),1.171(t,J=7.20Hz,3H);LC-MS(ESI+):m/zcalculatedforC22H16F5N2O2(M+H)+:435.11,found435.03.化合物D3-65的结构确证数据:(Z)-1-(3-(3,4-difluorophenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)hex-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.844(d,J=8.40Hz,2H),7.743(d,J=8.60Hz,2H),7.685-7.633(m,1H),7.596-7.528(m,1H),7.224-7.157(m,1H),7.088(s,1H),6.682(d,J=11.76Hz,1H),6.626(d,J=11.76Hz,1H),2.590(q,J=7.24Hz,2H),1.081(t,J=7.24Hz,3H)实施例56、制备化合物D3-67制备方法参见图56。(Z)-methoxymethyl4-(3-(3,4-difluorophenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)-4-oxobut-2-enoate1H-NMR(400MHz,CDCl3)δ(ppm)7.800(d,J=8.48Hz,2H),7.740(d,J=8.60Hz,2H),7.704-7.652(m,1H),7.571-7.359(m,1H),7.238-7.171(m,1H),6.876(d,J=11.90Hz,1H),6.369(d,J=11.90Hz,1H),5.263(s,2H),3.395(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)182.50,164.42,152.12,152.04,151.91,151.888,150.35,149.55,149.38,149.34,142.90,140.42,140.39,130.49(q,J=33.0Hz,C),128.81(q,J=4.0Hz,C),127.25,126.00(q,J=4.0Hz,CH),125.83,123.93(q,J=271.0Hz,C),122.04(q,J=3.0Hz,C),117.80(d,J=18.0Hz,CH),114.93(d,J=18.0Hz,CH),110.77,91.61,57.96.LC-MS(ESI+):m/zcalculatedforC22H16F5N2O4(M+H)+:467.10,found467.46实施例57、制备化合物D3-69和化合物D3-70制备方法参见图57。粗品经过硅胶柱纯化(洗脱体系石油醚:乙酸乙酯/4:1-2:1)得到3%产率的D3-69和67%产率的D3-70。化合物D3-69的结构确证数据:(E)-1-(3-(3,4-difluorophenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)-3-methylpent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.744(d,J=8.44Hz,2H),7.719-7.686(m,1H),7.620-7.572(m,1H),7.562(d,J=8.52Hz,2H),7.403(s,1H),7.277-7.210(m,1H),4.029(s,1H),2.287(s,3H),1.253(s,3H);LC-MS(ESI+):m/zcalculatedforC22H16F5N2O2(M+H)+:435.11,found435.38;化合物D3-70的结构确证数据:1H-NMR(400MHz,CDCl3)δ(ppm)7.720(d,J=8.32Hz,2H),7.695-7.667(m,1H),7.589(d,J=8.32Hz,2H),7.579-7.564(m,1H),7.237(s,1H),7.237-7.194(m,1H),6.574(d,J=1.48Hz,1H),2.295(s,3H),2.116(d,J=1.48Hz,3H);13C-NMR(100MHz,CDCl3)δ(ppm)205.64,179.03,158.25,152.02(d,J=3.0Hz,C),150.45,149.66,142.96,141.28,133.83,130.83-128.85(m),126.08(q,J=4.0Hz,CH),125.25,123.15,122.55,122.09(q,J=4.0Hz,C),117.91(d,J=18.0Hz,CH),115.06(d,J=19.0Hz,CH),109.90,28.18,20.83;LC-MS(ESI+):m/zcalculatedforC22H16F5N2O2(M+H)+:435.11,found435.38实施例58、制备化合物D3-71制备方法参见图58。(Z)-1-(3-(3,4-difluorophenyl)-1-phenyl-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.698-7.646(m,1H),7.610(d,J=7.24Hz,2H),7.559-7.528(m,1H),7.492-7.412(m,3H),7.214-7.147(m,1H),7.100(s,1H),6.621(d,J=11.84Hz,1H),60506(d,J=11.84Hz,1H),2.263(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)199.17,182.31,151.75(t,J=12.0Hz,C),149.62,149.27(t,J=12.0Hz,C),140.49,140.22,136.87,134.80,129.25-128.79(m),125.62,125.56,121.94(q,J=3.0Hz,CH),117.64(d,J=17.0Hz,CH),114.81(d,J=20.0Hz,CH),110.49,109.51,29.90;LC-MS(ESI+):m/zcalculatedforC20H15F2N2O2(M+H)+:353.11,found353.05.实施例59、制备化合物D3-73、化合物D3-74制备方法参见图59。粗品经过硅胶柱纯化(洗脱剂为石油醚:乙酸乙酯/3:1-1:1-二氯甲烷:甲醇/20:1)得到纯品D3-73和D3-74。化合物D3-73的结构确证数据:(Z)-1-(3-(6-bromopyridin-3-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)8.778(d,J=2.28Hz,1H),8.052(dd,J=8.32Hz,J=2.48Hz,1H),7.832(d,J=8.48Hz,2H),7.761(d,J=8.48Hz,2H),7.556(d,J=8.28Hz,1H),7.162(s,1H),6.710(d,J=11.68Hz,1H),6.651(d,J=11.68Hz,1H),2.318(s,3H);LC-MS(ESI+):m/zcalculatedforC20H14BrF3N3O2(M+H)+:464.02,found464.29化合物D3-74的结构确证数据:(Z)-2-bromo-5-(5-(4-oxopent-2-enoyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)pyridine1-oxide1H-NMR(400MHz,CDCl3)δ(ppm)8.954(s,1H),7.819(d,J=8.48Hz,2H),7.758(d,J=8.48Hz,2H),7.724-7.648(m,1H),7.568(d,J=8.36Hz,1H),7.175(s,1H),6.704(d,J=11.68Hz,1H),6.666(d,J=11.68Hz,1H),2.311(s,3H);LC-MS(ESI+):m/zcalculatedforC20H14BrF3N3O3(M+H)+:480.02,found480.23.实施例60、制备化合物D3-76制备方法参见图60。粗品经过硅胶柱分离纯化(洗脱剂体系为石油醚:乙酸乙酯/3:1-2:1)得到纯品。(Z)-1-(3-(3,4-difluorophenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.722-7.670(m,1H),7.560-7.529(m,1H),7.251-7.184(m,1H),7.116(s,1H),7.025(d,J=10.52Hz,1H),6.421(d,J=10.52Hz,1H),2.005(s,3H);LC-MS(ESI+):m/zcalculatedforC14H11F2N2O2(M+H)+:277.18,found277.33.实施例61、制备化合物D3-77和化合物D3-77’制备方法参见图61。反应后的粗品经过硅胶柱纯化(洗脱剂石油醚:乙酸乙酯/1:1-1:3-1:20)得到两者的混合物。所得产物未经过进一步纯化直接用于测活。两者的比例经过核磁共振测定所得。1H-NMR(400MHz,CDCl3)δ(ppm)7.937(d,J=8.08Hz,0.86H),7.889(d,J=8.12Hz,2H),7.828(d,J=8.40Hz,2H),7.758-7.737(m,2.95H),7.594(d,J=8.44Hz,0.92H),7.519-7.451(m,3.71H),7.171(s,1H),7.124(d,J=15.60Hz,0.37H),6.716(d,J=11.72Hz,1H),6.629(d,J=11.72Hz,1H),3.751-3.722(m,11H),2.418(s,1.09H),2.306(s,3H);LC-MS(ESI+):m/zcalculatedforC26H23F3N3O4(M+H)+:498.16,found498.46.实施例62、制备化合物D3-78制备方法参见图62。(E)-1-(1-(4-(morpholine-4-carbonyl)phenyl)-3-phenyl-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.875(d,J=7.16Hz,2H),7.524(s,4H),7.465-7.383(m,4H),7.452(d,J=15.68Hz,1H),7.095(d,J=15.68Hz,1H),3.716-3.640(m,8H),2.399(s,3H);LC-MS(ESI+):m/zcalculatedforC25H24N3O4(M+H)+:430.18,found430.11.实施例63、制备化合物D3-79制备方法参见图63。(Z)-di-tert-butyl(4-(5-(4-oxopent-2-enoyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)phenyl)phosphate1H-NMR(400MHz,CDCl3)δ(ppm)7.830-7.782(m,4H),7.738(d,J=8.32Hz,2H),7.283(d,J=8.48Hz,2H),7.112(s,1H),6.714(d,J=11.72Hz,1H),6.615(d,J=11.72Hz,1H),2.302(s,3H),1.515(s,18H);LC-MS(ESI+):m/zcalculatedforC29H33F3N2O6P(M+H)+:593.20,found593.48.实施例64、制备化合物D3-80制备方法参见图64。(Z)-1-(3-(4-methoxyphenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.821(d,J=8.40Hz,2H),7.772(d,J=8.72Hz,2H),7.736(d,J=8.48Hz,2H),7.091(s,1H),6.958(d,J=8.72Hz,2H),6.704(d,J=11.76Hz,1H),6.592(d,J=11.76Hz,1H),3.838(s,3H),2.294(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)198.87,182.74,160.29,152.18,143.22,140.22,136.59,135.56,130.19(q,J=33.0Hz,C),127.26,127.26,126.19,125.96(q,J=4.0Hz,CH),124.33,124.05(q,J=270.0Hz,CF3),114.44,114.35,110.10,55.43,30.06;LC-MS(ESI+):m/zcalculatedforC22H18F3N2O3(M+H)+:415.13,found415.04.实施例65、制备化合物D3-81制备方法参见图65。(Z)-1-(3-(3,4-difluorophenyl)isoxazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.713-7.663(m,1H),7.589-7.541(m,1H),7.315-7.249(m,1H),7.221(s,1H),6.817(d,J=11.84Hz,1H),6.726(d,J=11.84Hz,1H),2.367(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)200.01,180.30,166.96,161.67,141.00,130.40,123.61(q,J=4.0Hz,C),118.36(d,J=18.0Hz,CH),116.30(d,J=19.0Hz,CH),105.77,29.65;LC-MS(ESI+):m/zcalculatedforC14H10F2NO3(M+H)+:278.06,found278.35.实施例66、制备化合物D3-82制备方法参见图66。与图43所用制备方法相同,所用m-CPBA的当量不同。1-(3-(3-(3,4-difluorophenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazole-5-carbonyl)oxiran-2-yl)ethanone1H-NMR(400MHz,CDCl3)δ(ppm)7.757(d,J=8.40Hz,2H),7.737-7.685(m,1H),7.606-7.584(m,1H),7.558(d,J=8.56Hz,2H),7.495(s,1H),7.278-7.212(m,1H),4.084(d,J=1.56Hz,1H),3.724(d,J=1.56Hz,1H),2.207(s,3H);LC-MS(ESI+):m/zcalculatedforC21H14F5N2O3(M+H)+:437.09,found437.38.实施例67、制备化合物D3-83a和化合物D3-83b制备方法参见图67。反应中也得到了D3-82b,旋干后的粗品经过三次硅胶柱梯度纯化(第一次:二氯甲烷:乙酸乙酯/50:1-30:1;第二次:石油醚:丙酮/3:1-2:1;第三次:石油醚:丙酮/2:1)得到两个化合物的纯品。化合物D3-83a的结构确证数据:(E)-1-(3-(3,4-dihydroxyphenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.695(d,J=8.00Hz,2H),7.529(d,J=8.04Hz,2H),7.449(d,J=15.76Hz,1H),7.345(s,1H),7.260(s,1H),7.231(d,J=7.76Hz,1H),7.101(d,J=15.60 Hz,1H),6.878(d,J=8.20Hz,1H),2.415(s,3H);LC-MS(ESI+):m/zcalculatedforC21H16F3N2O4(M+H)+:417.11,found417.44.化合物D3-83b的结构确证数据:(Z)-1-(3-(3,4-dihydroxyphenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.722(d,J=7.96Hz,2H),7.658(d,J=7.88Hz,2H),7.271(d,J=2.28Hz,1H),7.186-7.146(m,1H),6.971(s,1H),6.808-6.774(m,1H),6.659(d,J=11.76Hz,1H),6.574(d,J=11.76Hz,1H),2.289(s,3H);实施例68、制备化合物D3-84制备方法参见图68。(Z)-1-(1,3-bis(4-hydroxyphenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.669(d,J=8.40Hz,2H),7.340(d,J=8.56Hz,2H),7.262(s,1H),6.866(d,J=8.40Hz,2H),6.835(d,J=8.40Hz,2H),6.744(d,J=11.84Hz,1H),6.590(d,J=11.84Hz,1H).实施例69、制备化合物D3-85b制备方法参见图69。1H-NMR(400MHz,CDCl3)δ(ppm)7.846-7.818(m,4H),7.746(d,J=8.40Hz,2H),7.429(d,J=7.60Hz,2H),7.159(s,1H),6.721(d,J=12.0Hz,1H),6.618(d,J=12.0Hz,1H),4.730(s,2H),2.310(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)198.82,182.78,152.07,143.19,141.69,140.37,136.58,135.68,131.03,127.49,126.27,126.09(q,J=4.0Hz,CH),125.89,110.46,65.10,30.12.实施例70、制备化合物D3-86制备方法参见图70。(E)-1-(3-(3-fluoro-4-morpholinophenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.745(d,J=8.44Hz,2H),7.600-7.556(m,4H),7.480(d,J=15.60Hz,1H),7.341(s,1H),7.116(d,J=15.60Hz,1H),6.986(t,J=8.68Hz,1H),3.887(t,J=4.40Hz,4H),3.151(t,J=4.40Hz,4H),2.417(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)197.30,179.24,155.79(d,J=245.0Hz,C),151.59,143.07,140.87,140.67(d,J=8.0Hz,C),139.00,134.40,126.22,126.16(d,J=3.0Hz,CH),125.94(d,J=8.0Hz,C),123.83,122.13(d,J=4.0Hz,CH),118.89(d,J=3.0Hz,CH),113.96(d,J=23.0Hz,CH),110.67,67.08,50.86,29.50;LC-MS(ESI+):m/zcalculatedforC25H22F4N3O3(M+H)+:488.16,found488.47.实施例71、制备化合物D3-87制备方法参见图71。(E)-1-(3-(6-morpholinopyridin-3-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)8.657(d,J=2.04Hz,1H),8.010(dd,J=8.80Hz,J=2.28Hz,1H),7.740(d,J=8.40Hz,2H),7.580(d,J=8.28Hz,2H),7.478(d,J=15.64Hz,1H),7.325(s,1H),7.113(d,J=15.64Hz,1H),6.698(d,J=8.88Hz,1H),3.836(t,J=4.72Hz,4H),3.583(t,J=4.72Hz,4H),2.413(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)197.37,179.26,159.61,150.63,145.88,143.08,140.74,138.96,135.13,134.42,126.16(q,J=4.0Hz,CH),123.71,117.51,110.10,106.68,66.83,45.55,29.51;LC-MS(ESI+):m/zcalculatedforC24H22F3N4O3(M+H)+:471.16,found471.45.实施例72、制备化合物D3-88b制备方法参见图72。(Z)-methyl3-(3-(3,4-difluorophenyl)-5-(4-oxopent-2-enoyl)-1H-pyrazol-1-yl)benzoate1H-NMR(400MHz,CDCl3)δ(ppm)8.28(s,1H),8.13(d,J=8.0Hz,1H),7.86-7.84(m,1H),7.71-7.66(m,1H),7.55-7.60(m,2H),7.25-7.18(m,1H),7.10(s,1H),6.685(d,J=12.0Hz,1H),6.585(d,J=12.0Hz,1H),3.94(s,2H),2.30(s,3H).实施例73、制备化合物D3-89制备方法参见图73。(Z)-1-(3-(3,4-difluorophenyl)-1-(3-(hydroxymethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.693-7.646(m,1H),7.587(s,1H),7.544-7.526(m,2H),7.484-7.417(m,2H),7.233-7.167(m,1H),7.090(s,1H),6.632(d,J=11.80Hz,1H),6.526(d,J=11.80Hz,1H),4.755(s,2H),2.290(s,3H)实施例74、制备化合物D3-90制备方法参见图74。(Z)-1-(5-(4-methoxyphenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.606(d,J=8.36Hz,2H),7.444(d,J=8.12Hz,2H),7.222(d,J=12.08Hz,1H),7.104(d,J=8.64Hz,2H),6.973(s,1H),6.840(d,J=8.64Hz,2H),6.517(d,J=12.08Hz,1H),3.778(s,3H),2.343(s,3H)实施例75、制备化合物D3-91、化合物D3-92、化合物D3-93制备方法参见图75。图75中所得到的中间体为吡唑啉和消除产物的混合物372mg,比例为1:0.17,反应未经进一步分离,直接投入下一步反应,所用间氯过氧苯甲酸为503mg,反应经过二氯甲烷和饱和碳酸氢钠溶液后处理后,硅胶柱梯度纯化(石油醚:乙酸乙酯/7:1-3:1-2:1-1:1)依次得到三种化合物D3-91,D3-92和D3-93的纯品。化合物D3-92的结构确证数据:(Z)-1-(1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)8.031(d,J=2.56Hz,1H),7.871(d,J=8.48Hz,2H),7.742(d,J=8.56Hz,2H),7.261(d,J=12.08Hz,1H),7.057(d,J=2.56Hz,1H),6.570(d,J=12.08Hz,1H),2.370(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)202.68,185.06,152.48,142.04,141.48,130.33,129.74(q,J=33.0Hz,C),129.46,129.34,129.15,127.06(q,J=4.0Hz,CH),123.82(q,J=270.0Hz,CF3),119.73,109.62,29.73;LC-MS(ESI+):m/zcalculatedforC15H12F3N2O2(M+H)+:309.09,found309.36.化合物D3-91的结构确证数据:(Z)-1-(5-(5-methylfuran-2-yl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-3-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.745(d,J=8.48Hz,2H),7.591(d,J=8.16Hz,2H),7.195(d,J=12.12Hz,1H),7.144(s,1H),6.529(d,J=12.12Hz,1H),6.042(d,J=3.20Hz,1H),5.982(d,J=2.44Hz,1H),2.376(s,3H),2.252(s,3H)化合物D3-93的结构确证数据:(2Z,2'Z)-1,1'-(1-(4-(trifluoromethyl)phenyl)-1H-pyrazole-3,5-diyl)bis(pent-2-ene-1,4-dione)1H-NMR(400MHz,CDCl3)δ(ppm)7.765(d,J=8.52Hz,2H),7.690(d,J=8.48Hz,2H),7.305(s,1H),7.058(d,J=12.04Hz,1H),6.588(s,2H),6.510(d,J=12.04Hz,1H),2.257(s,3H),2.187(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)201.93,198.42,184.65,183.16,150.52,142.50,141.38,140.74,135.91,130.74(q,J=33.0Hz,C),129.01,125.93(q,J=4.0Hz,CH),125.79,123.63(q,J=270.0Hz,CF3),113.10,29.78,29.36;LC-MS(ESI+):m/zcalculatedforC20H16F3N2O4(M+H)+:405.11,found405.40.实施例76、制备化合物D3-94制备方法参见图76。(Z)-1-(3-(4-(prop-2-yn-1-yloxy)phenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.830-7.708(m,6H),7.091(s,1H),7.029(d,J=8.64Hz,2H),6.702(d,J=11.72Hz,1H),6.598(d,J=11.72Hz,1H),4.721(d,J=2.32Hz,2H),2.544(t,J=2.32Hz,1H),2.293(s,3H)。实施例77、制备化合物D3-95制备方法参见图77。(Z)-1-(3-(3,4-difluorophenyl)-1-(4-(prop-2-yn-1-yloxy)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.769-7.627(m,1H),7.536-7.514(m,3H),7.211-7.144(m,1H),7.070-7.030(m,3H),6.622(d,J=11.80Hz,1H),6.508(d,J=11.80Hz,1H),4.725(d,J=2.24Hz,2H),2.549(t,J=2.24Hz,1H),2.265(s,3H);13C-NMR(100MHz,CDCl3)δ(ppm)199.11,182.42,157.79,151.81(t,J=13.0Hz,C),149.49,149.38,149.34,149.21,140.48,136.71,135.06,134.14,129.27(q,J=4.0Hz,C),127.01,126.94,121.93(q,J=4.0Hz,CH),117.78,117.60,115.02(q,J=10.0Hz,CH),110.27,109.28,78.25,76.04,69.60,56.15,29.34实施例78、制备化合物D3-96制备方法参见图78。(Z)-1-(4-bromo-3-(3,4-difluorophenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.840(d,J=8.44Hz,2H),7.785-7.745(m,1H),7.756(d,J=8.52Hz,2H),7.677-7.641(m,1H),7.297-7.220(m,2H),6.706(d,J=11.76Hz,1H),6.616(d,J=11.76Hz,1H),2.324(s,3H);LC-MS(ESI+):m/zcalculatedforC21H13BrF5N2O2(M+H)+:499.01,found498.94.实施例79、制备化合物D3-97制备方法参见图79。(Z)-1-(3-(3,4-difluorophenyl)-4-iodo-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.813(d,J=8.52Hz,2H),7.750(d,J=8.64Hz,2H),7.695-7.643(m,1H),7.596-7.574(m,1H),7.291-7.224(m,1H),6.707(d,J=11.80Hz,1H),6.581(d,J=11.80Hz,1H),2.321(s,3H)实施例80、制备化合物D3-101制备方法参见图80。(Z)-1-(4-(but-3-yn-1-yloxy)-3-(3,4-difluorophenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5 -yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)8.021-7.968(m,1H),7.878-7.847(m,1H),7.770(d,J=8.48Hz,2H),7.744(d,J=8.48Hz,2H),7.221(q,J=4.64Hz,1H),6.851(d,J=11.80Hz,1H),6.581(d,J=11.80Hz,1H),3.886(t,J=6.12Hz,2H),2.785(t,J=6.12Hz,2H),2.314(s,3H);LC-MS(ESI+):m/zcalculatedforC25H18F5N2O3(M+H)+:489.12,found489.09.实施例81、制备化合物D3-102制备方法参见图81。(Z)-1-(3-(3,4-difluorophenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)-4-(4-nitrophenyl)but-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)8.312(d,J=9.68Hz,2H),8.074(d,J=8.68Hz,2H),7.719-7.632(m,5H),7.555-7.534(m,1H),7.250(t,J=8.44Hz,1H),7.209(s,1H),7.172(d,J=11.68Hz,1H),7.118(d,J=11.68Hz,1H);LC-MS(ESI+):m/zcalculatedforC26H15F5N3O4(M+H)+:528.10,found528.15.实施例82、制备化合物D3-106制备方法参见图82。(Z)-1-(3-(3,4-difluorophenyl)-1-(prop-2-yn-1-yl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.642-7.590(m,1H),7.540-7.479(m,1H),7.212-7.145(m,1H),6.990(s,1H),6.766(d,J=11.92Hz,1H),6.582(d,J=11.92Hz,1H),5.406(d,J=2.44Hz,2H),2.390(t,J=2.44Hz,1H),2.343(s,3H)实施例83、制备化合物D3-107制备方法参见图83。(Z)-4-(3-(3,4-difluorophenyl)-5-(4-oxopent-2-enoyl)-1H-pyrazol-1-yl)benzenesulfonamide1H-NMR(400MHz,CDCl3)δ(ppm)8.052(d,J=4.68Hz,2H),7.877(d,J=4.68Hz,2H),7.689-7.646(m,1H),7.568-7.519(m,1H),7.260-7.189(m,1H),7.087(s,1H),6.714(d,J=11.60Hz,1H),6.656(d,J=11.60Hz,1H),4.837(s,2H),2.323(s,3H);LC-MS(ESI+):m/zcalculatedforC20H16F2N3O4S(M+H)+:432.08,found432.01.实施例84、制备化合物D3-108制备方法参见图84。(Z)-1-(3-(3-hydroxyphenyl)-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.782(d,J=8.40Hz,2H),7.706.(d,J=8.44Hz,2H),7.339(d,J=7.68Hz,1H),7.301(s,1H),7.262(d,J=7.84Hz,1H),7.100(s,1H),6.807(d,J=8.04Hz,1H),6.697(d,J=11.76Hz,1H),6.604(d,J=11.74Hz,1H),2.304(s,3H)实施例85、制备化合物D3-109制备方法参见图85。(Z)-1-(3-(4-chlorophenyl)-4-methyl-1-(4-(trifluoromethyl)phenyl)-1H-pyrazol-5-yl)pent-2-ene-1,4-dione1H-NMR(400MHz,CDCl3)δ(ppm)7.732(d,J=8.36Hz,2H),7.645(d,J=8.48Hz,2H),7.558(d,J=8.48Hz,2H),7.455(d,J=8.52Hz,2H),6.955(d,J=15.92Hz,1H),6.879(d,J=15.92Hz,1H),2.399(s,3H),2.225(s,3H);LC-MS(ESI+):m/zcalculatedforC22H17ClF3N2O2(M+H)+:433.09,found433.03.第二部分、化合物的药效实验实施例86、化合物的抗菌活性测定1、化合物对肺炎链球菌、金黄色葡萄球菌,粪肠球菌的活性测定所有化合物的最小抑菌浓度(MIC)测定均是参照CLSI(ClinicalAndLaboratoryStandardsInsititute)提供的标准方法进行,本实验采用的是微量液体稀释法测定最小抑菌浓度。1)所有待测化合物用DMSO溶解至20mg/ml,作为储存液保存在-20℃冰箱中,用Mueller-HintonBroth(MHB)培养基将药物稀释成128μg/ml的浓度。2)培养基的选用:金黄色葡萄球菌和粪肠球菌使用MHB培养基,肺炎链球菌使用含有2.5%裂解的马血的MHB培养基。3)接种物的制备:将上述三种细菌在MHB培养基中分别培养至对数生长期,然后稀释到106CFU/ml。4)MIC板制备:选用无菌96孔板,在板子A排的第1至第11孔加药液,每孔200μl,浓度为128μg/ml,第12孔不加药作为生长对照。每次试验中设置万古霉素的对照,A排中万古霉素的浓度为8μg/ml。5)使用排枪在B到H排。预先加好100μl不含抗生素的MHB培养基,从第一排中吸取100μl药液,加入到第2排中,并吸打混匀,每孔吸出100μl移至第3排,吸打混匀,依次类推,稀释至最低浓度2μg/ml(第7排),此时第8排中有培养基200μl,应吸出100μl,保持所有孔中体积为100μl。123456789101112ABCDEFGH6)用排枪向含有稀释好药液的96孔板中加100μl制备好的接种物,置在37℃普通空气孵箱中,肺炎链球菌的孵育时间为20~24h,试验葡萄球菌和肠球菌对万古霉素的药敏试验需孵24h。此时,第1孔至第11孔的药物终浓度分别为64、32、16、8、4、2、1μg/ml。7)结果判断:以在小孔内完全抑制细菌生长的最低药物浓度为MIC(μg/ml)。当阳性对照孔(即不含抗生素)内细菌明显生长试验才有意义。8)具体结果见下表:测试所根据的原理是:化合物浓度等倍数稀释,根据低浓度下细菌生长,高浓度下细菌不生长的原则得到范围值。具体MIC数值的测量需要更精确梯度的设计,考虑到成本因素,暂时现提供范围值。注:Streptococcuspneumoniae表示肺炎链球菌;Staphylcoccusaureus表示金黄色葡萄球菌;Enterococcusfaecalis表示为粪肠球菌。2、部分化合物对多重耐药肺炎链球菌(MRSP)、耐甲氧西林金黄色葡萄球菌(MRSA),耐万古霉素粪肠球菌(VRE)的活性测定1)多重耐药肺炎链球菌,耐甲氧西林金黄色葡萄球菌,耐万古霉素粪肠球菌均是临床分离菌株。2)化合物对这些耐药菌的MIC测定的方法同2.1,同时对于这些耐药菌设定了氨苄西林和万古霉素的对照。3)具体结果见下表:表2注:TH2784,TH2882,TH2889,TH2863:临床上分离的耐药肺炎链球菌株;MRSA:耐甲氧西林金黄色葡萄球菌;表3注:MRSP:多重耐药的肺炎链球菌;MDEF:多重耐药的粪肠球菌;VRE:耐万古霉素的粪肠球菌;MRSA:耐甲氧西林金黄色葡萄球菌;Vancomycin:万古霉素;Ampicillin:氨苄西林。当前第1页1 2 3
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1