靶向ALK的PROTAC及其应用的制作方法
本发明涉及靶向alk的protac及其应用,属于抗肿瘤药物
技术领域:
。
背景技术:
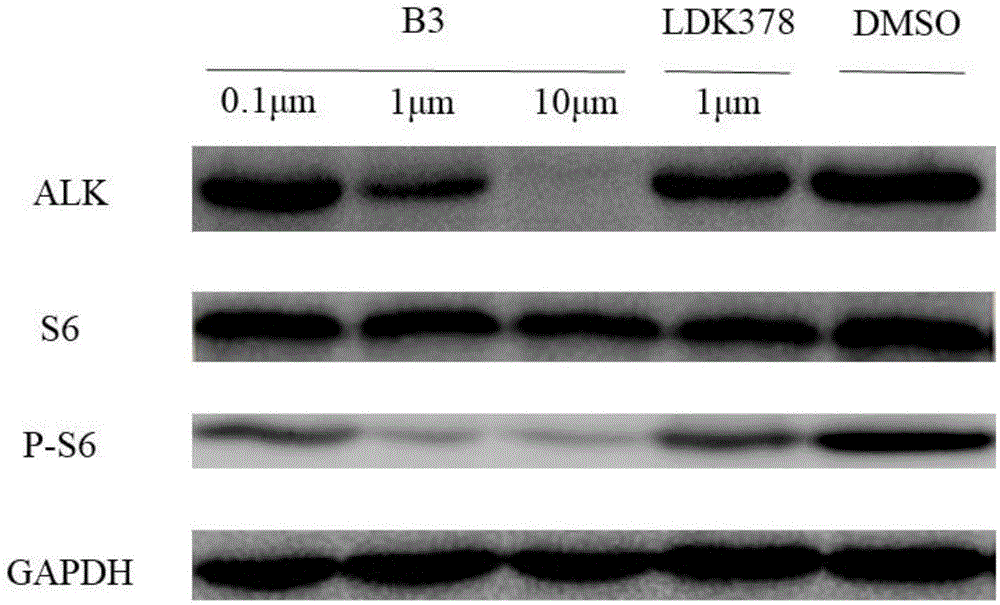
:蛋白水解靶向嵌合体(proteolysis-targetingchimeras,protac)是具有两个异功能的配体通过linker连接的化合物:一个配体靶向于目的蛋白(poi),而另一个配体特异性地募集e3连接酶。当protac结合e3连接酶和目的蛋白时形成了三元复合物,通过劫持e3连接酶,protac使poi呈现出有利的空间位置以促进其泛素化,从而选择性地降低靶蛋白的水平。这种方法的优点是protac可以催化样的多轮降解靶蛋白,这是protac分子与小分子抑制剂最大的不同。间变性淋巴瘤激酶(anaplasticlymphomakinase,alk)是一种受体酪氨酸激酶,与血液、间质和实体三大类型肿瘤相关。约3-7%的非小细胞肺癌(nsclc)患者体内肿瘤染色体eml4基因外显子与alk基因外显子融合,形成eml4-alk融合酪氨酸激酶,eml4-alk融合变异体具有高度的致癌性,且alk在多种肿瘤细胞中高表达。因此,alk成为一个极具吸引力的癌症治疗靶点。ldk378,通用名:色瑞替尼,商品名:zykadia,是目前已经上市的alk抑制剂,适用于有间变性淋巴瘤激酶(alk)-阳性转移对克唑替尼进展或不能耐受的非小细胞肺癌(nsclc)患者的治疗。其主要成分的化学结构式为:对ldk378进行结构改造,将ldk378与e3连接酶配体结合形成protac,可以催化样的多轮降解靶蛋白,选择性地降低靶蛋白的水平。专利cn104736569a公开了通过e3泛素连接酶增强靶蛋白及其他多肽降解的化合物和方法,主要是采用vhl配体作为e3连接酶配体,将靶向的蛋白质/多肽引至e3连接酶,以进行泛素化和随后的蛋白酶体降解。但是,发明人研究发现,ldk378与vhl配体连接形成的protac分子与阳性药物ldk378相比,对alk的激酶活性急剧下降,且抗肿瘤活性下降。技术实现要素:针对以上缺陷,本发明解决的技术问题是提供一种靶向alk的protac分子。本发明的靶向alk的protac分子,其结构式如式ⅰ所示:其中,l为连接体基团。本发明还提供本发明所述的化合物在制备抗肿瘤药物中的应用。本发明的化合物,可用于制备抗肿瘤药物。尤其适用于制备治疗肺癌或者宫颈癌的药物。本发明还提供了药物组合物,它包含治疗有效量的本发明所述的化合物以及至少一种可药用的载体。本发明还提供本发明所述的化合物在制备荧光示踪剂或肿瘤标志物中的应用。本发明化合物,具有荧光,可以制备成荧光示踪剂或肿瘤标志物,可用于追踪化合物分子在细胞内或体内的分布。与现有技术相比,本发明具有如下有益效果:本发明选用crbn的配体泊马度胺及其衍生物为e3连接酶配体,通过不同种类、不同链长的linker将alk抑制剂ldk378与e3连接酶偶联,成功制备得到了靶向alk的protac分子,能有效靶向于目标蛋白,并降低细胞中alk的含量,同时具有较好的体内体外抗肿瘤活性,对正常细胞毒性较低,符合高效低毒的特征。此外,该分子具有荧光,可以用于追踪化合物分子在细胞内或体内的分布。附图说明图1为化合物b3处理过h3122细胞12h后alk及相关蛋白含量变化图。图2为化合物b3处理的h3122细胞的pi/annexinv双染实验。图3为化合物b3的波长-吸收曲线。图4为化合物b3在420nm激发光下的不同波长发射荧光强度。图5为化合物b3分子在细胞内的分布图。图6为不同细胞加入化合物b3后细胞内的荧光强度图。图7为肿瘤体积随给药时间的变体曲线。图8为裸鼠体重随给药时间的变体曲线。具体实施方式本发明的靶向alk的protac分子,其结构式如式ⅰ所示:其中,l为连接体基团。优选的,l为其中,*端连接色瑞替尼哌啶环中的n,z为-ch2-、-nh-、-o-、-s-、z1为-ch2-、-nh-、-o-、-s-、z2为-ch2-、-nh-、-o-或-s-,m0为0~15中任一整数,m为0~15中任一整数,m1为0~8中任一整数,m2为0~8中任一整数,m3为0~8中任一整数,m4为0~15中任一整数,m5为0~15中任一整数;n为1~3中任一整数;m为h或1~8的饱和烷烃或环烷烃。优选的,z为z1为-nh-、z2为-ch2-或-o-。优选的,m0为1或2。更优选的,z为优选的,z1为-nh-、作为优选方案,z2为-o-,m1为1,m2为1。优选的,其结构式如b2、b3、c2、d1或g1所示:本发明的化合物,可用于制备抗肿瘤药物。尤其适用于制备治疗肺癌或者宫颈癌的药物。本发明的化合物可以单独使用,也可与可药用的载体或赋形剂一起以药物组合物的形式使用,当以药物组合物的形式使用时,通常将治疗有效量的本发明化合物以及一种或多种可药用载体或稀释剂结合制成适当的施用形式或剂量形式。因此,本发明还提供了药物组合物,它包含治疗有效量的本发明所述的化合物以及至少一种可药用的载体。本发明化合物的药用组合物,可以以下方面的任意方式施与:口服、喷雾吸入、直肠给药、鼻腔给药、阴道给药、局部给药、非肠道给药如皮下、静脉、肌内、腹膜内、销内、心室内、胸骨内或颅内注射或输入,或借助一种外植的储器用药,其中优选口服、肌注、腹膜内或静脉内用药方式。本发明化合物,具有荧光,可以制备成荧光示踪剂或肿瘤标志物,可用于追踪化合物分子在细胞内或体内的分布。下面结合实施例对本发明的具体实施方式做进一步的描述,并不因此将本发明限制在所述的实施例范围之中。实施例11、l系列分子的合成(i)乙二酰氯,dmf,rf,2h;(ii)三乙胺,dcm,rt,15h(iii)edci,hobt,dcm,rt,12h中间体p4的合成将原料丁二酸(118mg,0.1mmol)加入socl2中,加入2滴dmf,加热,回流反应2h。将反应液常温减压干燥得产物1.将产物1溶于无水dcm中,加入pomalidomide和triethylamine,在常温下反应15h。反应完成后,用水淬灭反应,用ea萃取三次,有机层用饱和食盐水洗两次,无水硫酸钠干燥,浓缩,用柱层析分离得中间体p4.1hnmr(400mhz,dmso)δ=8.47(d,j=8.1,1h),7.82(dd,j=8.4,7.4,1h),7.60(d,j=6.9,1h),5.14(dd,j=12.8,5.4,1h),2.91(ddd,j=19.1,12.4,5.4,2h),2.61(dt,j=15.3,5.5,4h),2.49–2.45(m,2h).hrms(dart-tof)calculatedforc17h16n3o7[m+h]+m/z374.0988,found374.0981.目标产物l4p的合成:将中间体p4溶于无水dcm中,加入edci和hobt,最后加入ldk378,常温反应12h。反应完成后,加入水,用ea萃取三次,有机层用饱和食盐水洗两次,用无水硫酸钠干燥,浓缩,用柱层析分离得目标产物,产率约为65%.1hnmr(400mhz,cdcl3)δ=9.64(s,1h),8.82(d,j=8.4,1h),8.57(d,j=8.2,1h),8.20(d,j=4.8,1h),8.15(s,1h),7.98(d,j=5.3,1h),7.96–7.92(m,1h),7.73–7.67(m,1h),7.65–7.59(m,1h),7.57–7.51(m,1h),7.32–7.27(m,1h),6.70(s,1h),4.96(dt,j=10.8,5.5,1h),4.80(d,j=12.8,1h),4.56(dp,j=12.1,6.0,1h),3.35–3.12(m,2h),3.02–2.64(m,9h),2.18(s,3h),1.91–1.49(m,8h),1.42–1.27(m,13h).13cnmr(101mhz,cdcl3)δ=171.95,170.64,169.42,169.38,167.79,166.75,155.48,137.85,136.26,134.62,131.32,131.19,126.86,125.33,125.05,123.69,123.29,118.38,115.47,110.91,71.71,55.54,49.28,46.20,42.88,38.32,32.79,32.29,31.40,22.67,22.27,18.96,15.37.hrms(dart-tof)calculatedforc45h50cln8o9s[m+h]+m/z913.3110,found913.3119.中间体p6的合成:中间体p6的合成步骤参照p4,但将原料换为己二酸,产率约为61%1hnmr(400mhz,dmso)δ=11.16(s,1h),9.72(s,1h),8.46(d,j=8.4,1h),7.96–7.77(m,1h),7.62(d,j=7.3,1h),5.15(dd,j=12.8,5.4,1h),2.90(ddd,j=16.7,13.8,5.3,1h),2.61(dd,j=14.6,3.9,1h),2.29–2.15(m,4h),2.12–2.01(m,1h),1.71–1.52(m,3h).13cnmr(101mhz,dmso)δ=174.98,173.26,172.34,170.29,168.09,167.15,136.97,136.56,131.95,126.86,118.80,117.55,49.36,36.64,34.12,31.40,24.79,24.63,24.56,22.45.hrms(dart-tof)calculatedforc19h20n3o7[m+h]+m/z402.1301,found402.1313.目标产物l6p的合成目标产物l6p的合成步骤参照l4p,但将原料换为p6,产率约为48%。1hnmr(400mhz,cdcl3)δ=9.55(s,1h),9.43(s,1h),8.82(d,j=8.4,1h),8.58(d,j=8.2,1h),8.44(s,1h),8.16(s,1h),8.00(s,1h),7.93(dd,j=8.0,1.5,1h),7.75–7.69(m,1h),7.62(dd,j=11.0,3.9,1h),7.58–7.52(m,1h),7.31–7.27(m,1h),6.69(s,1h),4.96(dd,j=11.7,4.8,1h),4.83(d,j=13.1,1h),4.63–4.48(m,1h),3.22(ddd,j=43.8,19.1,9.3,2h),2.99–2.59(m,5h),2.49(dt,j=14.1,6.8,4h),2.18(s,4h),1.90–1.48(m,10h),1.41–1.28(m,13h).13cnmr(101mhz,cdcl3)δ=172.00,170.79,170.74,169.16,167.89,166.68,155.42,144.93,138.42,137.82,136.44,134.62,131.30,131.13,127.76,126.87,125.29,124.97,123.66,123.21,121.00,118.49,115.35,110.91,71.68,55.51,49.30,46.47,42.63,38.38,37.69,33.45,32.95,32.30,31.39,24.96,24.72,22.68,22.27,22.18,18.96,15.37.hrms(dart-tof)calculatedforc47h53cln8o9sna[m+na]+m/z963.3242,found963.3241.中间体p7的合成:中间体p7的合成步骤参照p4,但将原料换为庚二酸,产率约为59%1hnmr(400mhz,dmso)δ=9.72(s,1h),8.47(d,j=8.4,1h),7.91–7.76(m,1h),7.61(d,j=7.2,1h),5.15(dd,j=12.8,5.4,1h),2.59(ddd,j=25.5,16.5,13.1,2h),2.19(dt,j=14.7,7.4,8h),1.75–1.59(m,2h),1.39–1.13(m,6h).13cnmr(101mhz,dmso)δ=175.04,173.25,172.44,170.28,168.12,167.15,137.00,136.56,131.94,126.81,118.77,117.50,49.36,40.58,40.37,40.16,39.96,39.75,39.54,39.33,36.82,34.23,31.40,28.65,28.56,25.01,24.81,22.44,21.81.hrms(dart-tof)calculatedforc20h22n3o7[m+h]+m/z416.1458,found416.1447.目标产物l7p的合成目标产物l7p的合成步骤参照l4p,但将原料换为p7,产率约为38%。1hnmr(400mhz,cdcl3)δ=9.43(s,1h),8.83(d,j=8.5,1h),8.57(d,j=8.3,1h),8.14(s,1h),7.94(dd,j=8.0,1.4,1h),7.78–7.68(m,1h),7.61(t,j=7.8,1h),7.56–7.52(m,1h),7.29(d,j=6.6,1h),6.69(s,1h),4.95(dd,j=12.2,5.3,1h),4.83(d,j=12.9,1h),4.55(dt,j=12.2,6.1,1h),4.00(d,j=13.4,1h),3.22(ddd,j=45.5,19.0,9.7,3h),3.00–2.58(m,5h),2.49(t,j=7.5,2h),2.44–2.36(m,2h),2.17(d,j=6.3,4h),1.67(dddd,j=36.1,23.0,14.9,7.6,15h),1.33(dt,j=30.8,14.6,13h).hrms(dart-tof)calculatedforc48h55cln8o9sna[m+na]+m/z977.3399,found977.3368.中间体p8的合成:中间体p8的合成步骤参照p4,但将原料换为辛二酸,产率约为61%1hnmr(400mhz,dmso)δ=9.69(s,1h),8.47(d,j=8.3,1h),7.87–7.79(m,1h),7.61(d,j=7.3,1h),5.14(dd,j=12.8,5.4,1h),2.90(ddd,j=16.7,13.7,5.2,1h),2.67–2.51(m,2h),2.46(t,j=7.4,2h),2.20–2.01(m,6h),1.69–1.36(m,6h).13cnmr(101mhz,dmso)δ=175.10,173.22,172.48,170.25,168.15,167.15,137.03,136.56,131.94,126.79,118.76,117.50,49.39,40.64,40.43,40.22,40.01,39.80,39.59,39.38,36.94,34.35,31.41,28.76,28.71,25.13,24.94.hrms(dart-tof)calculatedforc20h22n3o7[m+h]+m/z416.1458,found416.1447.中间体p9的合成:中间体p9的合成步骤参照p4,但将原料换为壬二酸,产率约为58%1hnmr(400mhz,dmso)δ=8.47(d,j=8.4,1h),7.91–7.78(m,1h),7.61(d,j=7.1,1h),5.15(dd,j=12.8,5.4,1h),2.90(ddd,j=16.7,13.8,5.2,1h),2.18(t,j=6.9,8h),1.55–1.42(m,10h).13cnmr(101mhz,dmso)δ=175.02,173.25,172.56,172.50,170.28,168.15,167.15,137.02,136.56,131.93,126.76,118.75,117.45,49.36,40.57,40.36,40.16,39.95,39.74,39.53,39.32,36.94,34.16,31.40,28.93,28.89,28.84,28.73,25.20,24.94,24.83,22.44,21.61.hrms(dart-tof)calculatedforc22h26n3o7[m+h]+m/z444.1771,found444.1762.目标产物l9p的合成目标产物l9p的合成步骤参照l4p,但将原料换为p9,产率约为45%。1hnmr(400mhz,cdcl3)δ=9.45(s,1h),8.83(d,j=8.5,1h),8.56(d,j=8.4,1h),8.13(s,1h),7.96–7.90(m,1h),7.76–7.68(m,1h),7.61(t,j=7.9,1h),7.55(d,j=7.0,1h),7.29(d,j=7.7,1h),6.69(s,1h),4.95(dd,j=12.4,5.4,1h),4.84(d,j=13.8,1h),3.27(dd,j=13.7,6.8,1h),3.19–3.10(m,1h),2.91(t,j=15.1,2h),2.84–2.73(m,2h),2.70–2.58(m,1h),2.47(t,j=6.5,1h),2.42–2.34(m,2h),2.18(s,3h),1.76(dd,j=58.1,42.8,18h),1.36(dt,j=15.7,7.2,11h).hrms(dart-tof)calculatedforc50h59cln8o9sna[m+na]+m/z1005.3112,found1005.3720.目标产物l10p的合成目标产物l10p的合成步骤参照l4p,但将原料换为p10,产率约为31%。1hnmr(400mhz,cdcl3)δ=9.45(s,1h),8.81(t,j=9.4,1h),8.68(s,1h),8.56(d,j=8.4,1h),8.13(s,1h),7.94(dd,j=8.0,1.3,2h),7.74–7.68(m,1h),7.60(t,j=7.4,1h),7.56–7.51(m,1h),7.28(t,j=5.1,1h),6.68(s,1h),4.96(dd,j=12.0,5.5,1h),4.84(d,j=13.0,1h),4.52(t,j=10.8,1h),4.01(dd,j=15.6,9.0,1h),3.25(dt,j=13.7,6.8,1h),3.14(t,j=12.3,1h),2.92(dd,j=24.8,9.5,3h),2.84–2.72(m,2h),2.63(t,j=12.0,2h),2.42(dt,j=14.9,6.9,5h),2.18(s,4h),1.86–1.49(m,16h),1.44–1.29(m,22h).13cnmr(101mhz,cdcl3)δ=172.39,172.03,171.73,170.79,169.21,167.89,166.72,137.26,136.43,134.63,131.34,131.15,126.81,125.26,123.71,118.41,115.34,77.34,77.02,77.02,76.70,71.68,55.59,49.32,42.58,38.41,38.01,33.50,32.43,31.42,29.26,29.12,28.98,25.27,22.18,18.95,15.37.hrms(dart-tof)calculatedforc51h61cln8o9sna[m+na]+m/z1019.3868,found1019.3871.中间体p11的合成:中间体p11的合成步骤参照p4,但将原料换为十一烷二酸,产率约为52%1hnmr(400mhz,dmso)δ=9.70(s,1h),8.47(d,j=8.3,1h),7.91–7.77(m,1h),7.61(d,j=6.8,1h),5.14(dd,j=12.8,5.4,1h),2.90(ddd,j=16.5,13.6,5.2,1h),2.65–2.53(m,1h),2.45(t,j=7.4,2h),2.16–2.02(m,6h),1.61(dd,j=14.1,7.0,2h),1.52–1.40(m,5h),1.37–1.24(m,10h).13cnmr(101mhz,dmso)δ=175.53,173.22,172.51,170.25,168.15,167.15,137.05,136.56,131.94,126.78,118.74,117.48,49.39,37.00,35.19,35.07,29.24,29.18,29.07,28.96,25.31.hrms(dart-tof)calculatedforc24h30n3o7[m+h]+m/z472.2084,found472.2065.目标产物l11p的合成目标产物l11p的合成步骤参照l4p,但将原料换为p11,产率约为29%。1hnmr(400mhz,cdcl3)δ=9.44(s,1h),8.83(d,j=8.4,1h),8.56(d,j=8.3,1h),8.14(s,1h),7.94–7.91(m,1h),7.74–7.68(m,1h),7.61(dd,j=11.4,4.4,1h),7.54(dd,j=7.3,0.6,1h),7.28(d,j=8.4,1h),6.68(s,1h),4.96(dd,j=12.2,5.3,1h),4.84(d,j=13.0,1h),4.59–4.48(m,1h),4.07–3.90(m,2h),3.26(dq,j=13.7,6.9,1h),3.16(dd,j=24.5,11.9,1h),2.98–2.73(m,4h),2.68–2.57(m,1h),2.46(t,j=7.4,2h),2.43–2.36(m,2h),2.17(s,3h),1.89–1.60(m,10h),1.38–1.30(m,21h).13cnmr(101mhz,cdcl3)δ=172.42,171.73,170.82,169.21,167.96,166.73,155.54,137.90,136.43,134.63,131.33,131.14,125.27,123.70,118.40,115.32,110.90,71.65,55.57,49.33,46.58,42.59,38.40,38.03,33.47,31.93,31.44,29.70,29.56,29.44,29.25,29.04,25.43,25.28,23.92,22.69,22.16,18.94,15.36,14.11.hrms(dart-tof)calculatedforc52h63cln8o9sna[m+na]+m/z1033.4025,found1033.4023.中间体p12的合成:中间体p12的合成步骤参照p4,但将原料换为十二烷二酸,产率约为46%1hnmr(400mhz,dmso)δ=9.68(s,1h),8.47(d,j=8.3,1h),7.88–7.78(m,1h),7.61(d,j=7.3,1h),5.14(dd,j=12.7,5.4,1h),2.45(t,j=7.4,2h),2.16(t,j=7.3,9h),1.61(dd,j=14.3,7.0,2h),1.55–1.39(m,9h),1.30(s,4h).hrms(dart-tof)calculatedforc25h32n3o7[m+h]+m/z486.2240,found486.2213.目标产物l12p的合成目标产物l12p的合成步骤参照l4p,但将原料换为p12,产率约为31%。1hnmr(400mhz,cdcl3)δ=8.56(d,j=8.0,1h),8.16(s,1h),8.03(s,1h),8.03(s,1h),7.94(d,j=7.9,1h),7.78(d,j=8.1,1h),7.67(d,j=8.3,1h),7.65–7.59(m,1h),7.56(s,1h),7.39–7.28(m,3h),6.76(s,1h),4.58–4.48(m,1h),3.82(d,j=13.2,2h),3.25(dd,j=13.9,6.8,3h),2.34(s,12h),2.16(s,3h),1.97(d,j=15.2,2h),1.68(s,7h),1.30(dd,j=14.5,8.4,13h),1.26(s,12h).hrms(dart-tof)calculatedforc53h65cln8o9sna[m+na]+m/z1047.4181,found1047.4181.2、a系列protac分子合成:中间体1的合成:以ldk378为原料(330mg,0.6mmol),溶于50ml甲醇,加入丙烯酸甲酯(78mg,0.9mmol),三乙胺(240mg,2.4mmol),常温搅拌8h,tlc检测反应进度。处理方法:先减压蒸馏除去甲醇,加入150ml乙酸乙酯,用水反洗有机溶液三次,用无水硫酸钠除水后减压浓缩,用pe/ea分离体系进行柱层析,得到白色固体,产率约为90%。1hnmr(400mhz,cdcl3)δ=9.49(s,1h),8.58(d,j=8.4,1h),8.15(s,1h),7.99(s,1h),7.93(d,j=8.0,1h),7.62(t,j=7.9,1h),7.53(s,1h),7.24(d,j=7.7,1h),6.80(s,1h),4.53(dt,j=12.1,6.0,1h),3.71(s,3h),3.32–3.21(m,1h),3.06(d,j=9.6,2h),2.77(d,j=6.7,2h),2.72–2.52(m,3h),2.15(s,4h),1.76(s,4h),1.34(dd,j=15.0,6.5,12h).13cnmr(101mhz,cdcl3)δ=157.52,155.39,155.33,144.72,138.52,134.65,131.25,127.46,126.87,124.91,123.71,123.09,120.63,110.82,105.72,71.43,55.45,54.30,53.90,51.71,37.94,32.77,22.27,18.93,15.37.hrms(dart-tof)calculatedforc32h43cln5o5s[m+h]+m/z644.2673,found644.2673.中间体2的合成:将中间体1(320mg,0.5mmol)溶于50ml甲醇和水1:1的混合液,加入氢氧化钠(200mg,5mmol),60℃下搅拌10h,反应液由澄清变浑浊,tlc监测反应。反应处理:先减压旋蒸除去甲醇,用稀盐酸调节水层ph至2。然后用ea萃取3次,取油层用饱和食盐水反洗1次,用无水硫酸钠干燥后减压浓缩,即得产物,白色粉末,产率约为95%。1hnmr(400mhz,cdcl3)δ=9.51(s,1h),8.58(d,j=8.4,1h),8.16(d,j=1.2,1h),8.03(d,j=3.2,1h),7.93(d,j=7.9,1h),7.62(dd,j=11.3,4.4,1h),7.58–7.51(m,1h),7.27(d,j=6.5,1h),6.75(d,j=37.6,1h),4.56(dt,j=11.1,5.6,1h),3.51–3.14(m,3h),2.81(ddd,j=47.2,24.3,12.3,3h),2.17(d,j=8.1,3h),1.34(dd,j=14.8,6.5,13h).hrms(dart-tof)calculatedforc31h41cln5o5s[m+h]+m/z630.2517,found630.2508.中间体3的合成:称取ldk378(560mg,1mmol),溶于50ml乙腈中,加入溴乙酸叔丁酯(195mg,1mmol),碳酸钾(414mg,3mmol),回流5h,用tlc监测反应。反应完全后加入30ml水淬灭反应,用100ml的ea萃取3次,后用水反洗3次。有机层用无水硫酸钠干燥,减压浓缩,用pe/ea作为流动相进行柱层析分离。得黄色固体,产率约为76%。1hnmr(400mhz,cdcl3)δ=9.49(s,1h),8.58(d,j=8.3,1h),8.14(s,1h),7.97(d,j=12.7,1h),7.92(d,j=7.8,1h),7.62(t,j=7.7,1h),7.54(s,1h),7.26(dd,j=13.1,5.4,1h),6.82(s,1h),4.51(dq,j=11.9,6.0,1h),3.34–3.22(m,1h),3.18(s,2h),3.09(d,j=10.9,2h),2.68(dd,j=15.9,7.8,1h),2.32(t,j=10.8,2h),2.16(s,3h),1.82(dt,j=34.1,11.0,4h),1.49(s,10h),1.34(dd,j=17.5,6.4,13h).hrms(dart-tof)calculatedforc34h47cln5o5s[m+h]+m/z672.2986,found672.2975.中间体4的合成:将中间体3溶于20ml三氟乙酸中,常温搅拌5h。反应完全后,在反应液中加入30mldcm,减压浓缩,最终得白色粉末,产率约为90%。1hnmr(400mhz,meod)δ=8.29(d,j=7.9,1h),8.23(s,1h),7.99(d,j=7.9,1h),7.70(t,j=7.7,1h),7.51(t,j=7.6,1h),7.40(s,1h),6.92(s,1h),4.72–4.59(m,1h),4.13(s,2h),3.82(t,j=18.3,2h),3.35(s,4h),3.13(t,j=11.7,1h),2.18(d,j=12.2,3h),2.13–1.92(m,4h),1.37–1.16(m,13h).hrms(dart-tof)calculatedforc30h39cln5o5s[m+h]+m/z616.2360,found616.2347.中间体a1-m的合成:在20mlnmp中,加入3-氟-n-(2,6-二氧代-3-哌啶基)邻苯二甲酰亚胺(165mg,0.6mmol)n-叔丁氧羰基-1,4-丁二胺(113mg,0.6mmol),dipea(129mg,1mmol),90℃下反应10h,tlc监测反应。反应完成后,在反应液中加入150mlea混匀后,首先用10%柠檬酸溶液洗2次,每次20ml,后用饱和nahco3溶液洗2次,每次20ml,最后用饱和食盐水洗2次后用无水硫酸钠干燥,减压浓缩后用pe/ea分离体系进行柱层析分离。产物为黄色固体,产率约为65%。1hnmr(400mhz,cdcl3)δ=8.27(d,j=30.0,1h),7.42(dd,j=8.5,7.1,1h),7.02(d,j=7.0,1h),6.82(d,j=8.5,1h),4.90–4.79(m,1h),3.23(t,j=6.9,2h),3.10(t,j=6.1,2h),2.87–2.63(m,4h),2.12–2.02(m,1h),1.67–1.49(m,4h),1.40(d,j=19.8,8h).hrms(dart-tof)calculatedforc22h28n4o6[m+h]+m/z444.2009,found444.2001.中间体a2-m的合成:该中间体的制备方法同a1-m,原料为n-叔丁氧羰基-1,6-己二胺。产率为63%1hnmr(400mhz,cdcl3)δ=8.30(s,1h),7.42(dd,j=8.3,7.3,1h),7.01(d,j=7.1,1h),6.80(d,j=8.6,1h),4.85(dd,j=12.0,5.4,1h),4.50(s,1h),3.19(t,j=7.0,2h),3.04(t,j=6.9,2h),2.89–2.55(m,4h),2.06(dt,j=6.7,3.0,1h),1.65–1.54(m,2h),1.48–1.24(m,15h).hrms(dart-tof)calculatedforc24h33n4o6[m+h]+m/z473.2400,found473.2391.中间体a3-m的合成:a3-m该中间体的制备方法同上,原料为n-叔丁氧羰基-1,8-辛二胺。产率为61%。1hnmr(400mhz,cdcl3)δ=8.30(s,1h),7.49(dd,j=8.4,7.3,1h),7.08(d,j=7.1,1h),6.88(d,j=8.5,1h),4.97–4.87(m,1h),3.26(t,j=6.9,2h),3.10(s,2h),2.98–2.66(m,4h),2.15(ddd,j=13.0,10.0,6.3,1h),1.70–1.62(m,2h),1.52–1.29(m,20h).hrms(dart-tof)calculatedforc26h36n4o6na[m+na]+m/z523.2533,found523.2512.目标产物a1的合成:将a1-m(90mg,0.2mmol)溶于5ml三氟乙酸中,常温搅拌5h。停止反应,在反应液中加入10mldcm并减压浓缩,得棕色油状物。在该油状物中加入10mldmf溶解,加入中间体2(63mg,0.1mmol),hatu(45.6mg,0.12mmol),dipea(38.7mg,0.3mmol)。常温反应15h,tlc监测反应进度。反应完成后用30ml水淬灭反应,用ea萃取水层4次,合并有机层,用饱和食盐水反洗2次,用无水硫酸钠干燥,减压浓缩,用dcm/meoh分离体系进行柱层析分离。产物为黄色粉末,产率约40%.1hnmr(400mhz,cdcl3)δ=9.45(s,1h),8.51(d,j=8.3,1h),8.09(s,1h),7.94(s,1h),7.90–7.78(m,1h),7.53(dd,j=18.5,11.2,3h),7.41(dd,j=15.8,8.3,1h),7.21–7.10(m,3h),7.00(d,j=7.1,1h),6.81(d,j=8.5,1h),6.70(s,1h),6.15(t,j=5.4,1h),4.94–4.71(m,1h),4.47(dt,j=11.9,5.9,1h),3.39–3.05(m,8h),2.96–2.29(m,12h),2.08(s,3h),1.84–1.53(m,10h),1.37–1.22(m,13h).13cnmr(101mhz,cdcl3)δ=171.83,171.20,169.59,168.67,167.55,157.42,155.37,146.87,144.80,138.48,136.23,134.62,132.48,131.30,128.11,127.17,124.92,123.59,123.16,120.82,116.72,111.63,111.06,110.06,105.96,71.88,60.40,55.49,53.86,48.91,42.16,31.71,26.93,26.59,22.23,15.37.hrms(dart-tof)calculatedforc48h59cln9o8s[m+h]+m/z956.3896,found956.3958.目标产物a2的合成:称取原料a2-m(240mg,0.5mmol),其它原料和操作方法同目标产物a1。得目标产物为黄色粉末,产率约为35%。1hnmr(400mhz,cdcl3)δ=9.46(s,1h),8.51(d,j=8.3,1h),8.09(d,j=6.0,1h),7.89(s,1h),7.85(dd,j=8.0,1.5,1h),7.79(s,1h),7.62(s,1h),7.56–7.49(m,1h),7.43–7.36(m,1h),7.16(d,j=7.9,1h),7.00(d,j=7.1,1h),6.78(d,j=8.5,1h),6.68(s,1h),6.24–6.08(m,1h),4.83(dd,j=12.3,5.3,1h),4.53–4.37(m,1h),3.29–3.07(m,8h),2.86–2.58(m,8h),2.42(t,j=6.3,2h),2.19(t,j=11.4,3h),2.13–2.01(m,5h),1.59(dt,j=13.9,6.8,3h),1.23(t,j=9.2,14h).13cnmr(101mhz,cdcl3)δ=171.98,171.40,169.60,168.91,167.67,157.47,155.38,155.19,146.92,144.97,138.48,136.12,134.62,132.54,131.27,127.82,126.98,124.87,123.61,123.10,121.15,116.57,111.41,110.96,109.98,105.80,71.59,60.40,55.48,54.14,53.81,48.98,42.50,39.12,37.58,32.38,31.93,31.52,29.69,29.44,29.06,26.81,26.67,22.80,22.69,22.12,18.90,15.36,14.20,14.12.hrms(dart-tof)calculatedforc50h63cln9o8s[m+h]+m/z984.4209,found984.4210.目标产物a3的合成:称取原料a3-m(250mg,0.5mmol),其它原料和操作方法同目标产物a1。得目标产物为黄色粉末,产率约为41%。1hnmr(400mhz,cdcl3)δ=9.53(s,1h),8.58(d,j=8.3,1h),8.20(s,1h),7.95(s,1h),7.92(dd,j=8.0,1.4,1h),7.76(s,1h),7.63–7.55(m,1h),7.51–7.44(m,1h),7.27–7.21(m,2h),7.07(d,j=7.1,1h),6.86(d,j=8.6,1h),6.73(s,1h),4.91(dd,j=12.2,5.3,1h),4.58–4.45(m,1h),3.36–3.22(m,5h),3.15(d,j=11.3,2h),2.95–2.64(m,7h),2.46(t,j=6.1,2h),2.27–2.09(m,6h),1.90–1.56(m,8h),1.40–1.29(m,19h).13cnmr(101mhz,cdcl3)δ=172.17,171.49,169.57,169.01,167.72,157.47,155.40,155.09,146.96,145.07,138.47,137.17,136.05,134.63,132.57,131.25,127.68,126.83,124.87,123.64,123.09,121.38,116.53,111.29,110.79,109.95,105.74,71.43,60.39,55.48,54.19,53.77,48.96,42.40,39.18,37.76,32.62,32.29,31.55,29.70,29.46,29.19,29.05,27.01,26.75,22.77,22.07,22.03,21.04,18.92,15.37,15.35,14.20.hrms(dart-tof)calculatedforc52h67cln9o8s[m+h]+m/z1012.4522,found1012.4512.目标产物a4的合成:该目标产物的原料为n-叔丁氧羰基-1,3-丙二胺以及中间体4,其它原料和操作方法同目标产物a1。得目标产物为黄色粉末,产率约为51%。1hnmr(400mhz,cdcl3)δ=9.52(s,1h),8.58(d,j=8.3,1h),8.15(d,j=6.5,1h),8.01(s,1h),7.93(dd,j=8.0,1.5,1h),7.66–7.59(m,1h),7.56(s,1h),7.49(dd,j=8.3,7.3,1h),7.35(t,j=6.3,1h),7.27–7.23(m,1h),7.09(d,j=7.0,1h),6.89(d,j=8.5,1h),6.77(s,1h),6.49(t,j=5.8,1h),4.98–4.85(m,1h),4.57(tp,j=12.2,6.1,1h),3.52–3.41(m,2h),3.36(q,j=6.5,2h),3.26(dt,j=13.7,6.8,1h),3.07(s,2h),2.96(d,j=11.4,2h),2.92–2.64(m,4h),2.33(dd,j=11.5,9.7,2h),2.15(s,3h),2.14–2.07(m,1h),1.91(dq,j=13.4,6.6,2h),1.83–1.64(m,5h),1.39–1.30(m,13h).13cnmr(101mhz,cdcl3)δ=171.13,171.04,169.41,168.34,167.55,157.48,155.36,146.72,144.74,138.51,137.24,136.17,134.63,132.63,131.29,127.88,127.31,124.90,123.61,123.11,120.90,116.45,111.59,111.13,110.26,105.85,71.82,62.06,55.48,55.13,48.92,40.08,37.66,36.47,32.95,31.43,29.79,29.70,22.77,22.29,18.94,15.37.hrms(dart-tof)calculatedforc46h55cln9o8s[m+h]+m/z928.3583,found928.3581.目标产物a5的合成:该目标产物的原料为中间体a1-m以及中间体4,其它原料和操作方法同目标产物a1。得目标产物为黄色粉末,产率约为49%。1hnmr(400mhz,cdcl3)δ=9.52(s,1h),8.59(d,j=8.2,1h),8.15(d,j=6.5,1h),8.00(s,1h),7.93(dd,j=8.0,1.5,1h),7.65–7.59(m,1h),7.58(s,1h),7.47(dt,j=12.5,6.3,1h),7.27–7.21(m,1h),7.07(t,j=6.9,1h),6.89(d,j=8.5,1h),6.78(s,1h),6.25(t,j=5.7,1h),4.89(dd,j=12.1,5.3,1h),4.57(tp,j=12.2,6.1,1h),3.43–3.22(m,5h),3.05(s,2h),2.96(d,j=11.4,2h),2.90–2.62(m,4h),2.32(t,j=10.9,2h),2.15(s,3h),1.82–1.64(m,9h),1.39–1.31(m,12h).13cnmr(101mhz,cdcl3)δ=171.05,170.65,169.55,168.40,167.54,157.49,155.36,155.33,146.85,144.75,138.50,137.32,136.19,134.64,132.51,131.28,127.89,127.37,124.89,123.61,123.11,120.95,116.61,111.59,111.27,110.06,105.85,71.90,62.09,55.48,55.09,48.90,42.19,38.42,37.65,32.92,31.41,29.70,27.43,26.64,22.78,22.28,18.93,15.37,14.20.hrms(dart-tof)calculatedforc47h57cln9o8s[m+h]+m/z942.3739,found942.3731.目标产物a6的合成:该目标产物的原料为中间体a2-m以及中间体4,其它原料和操作方法同目标产物a1。得目标产物为黄色粉末,产率约为43%。1hnmr(400mhz,cdcl3)δ=9.53(s,1h),8.59(d,j=8.1,1h),8.17(s,1h),7.98(s,1h),7.93(dd,j=8.0,1.5,1h),7.60(dd,j=13.0,5.9,2h),7.47(dd,j=8.3,7.3,1h),7.27–7.20(m,3h),7.07(d,j=7.0,1h),6.86(d,j=8.5,1h),6.77(s,1h),6.22(t,j=5.6,1h),4.98–4.84(m,1h),4.54(dq,j=12.1,6.1,1h),3.27(dtt,j=13.4,10.2,6.6,6h),3.05(s,2h),2.97(d,j=11.2,2h),2.91–2.63(m,5h),2.32(t,j=11.4,2h),2.15(s,3h),1.83–1.54(m,12h),1.37–1.30(m,15h).13cnmr(101mhz,cdcl3)δ=171.16,170.43,169.55,168.55,167.61,157.49,155.38,155.26,146.93,144.85,138.50,137.38,136.11,134.62,132.53,131.28,127.80,127.23,124.88,123.62,123.10,121.07,116.57,111.42,111.13,109.97,105.80,71.72,62.07,60.39,55.48,55.02,48.92,42.54,38.78,37.60,33.00,32.96,31.93,31.45,29.78,29.69,29.36,29.21,26.72,26.64,22.79,22.69,22.21,21.04,18.92,15.36,14.20,14.12.hrms(dart-tof)calculatedforc49h51cln9o8s[m+h]+m/z970.4052,found970.4038.目标产物a7的合成:该目标产物的原料为中间体a3-m以及中间体4,其它原料和操作方法同目标产物a1。得目标产物为黄色粉末,产率约为45%。1hnmr(400mhz,cdcl3)δ=9.53(s,1h),8.58(d,j=7.8,1h),8.17(s,1h),7.98(s,1h),7.93(dd,j=8.0,1.6,1h),7.65(s,1h),7.63–7.57(m,1h),7.47(dd,j=8.3,7.3,1h),7.27(s,1h),7.23(dt,j=8.3,4.1,1h),7.07(d,j=6.9,1h),6.87(dd,j=8.5,3.4,1h),6.77(s,1h),6.23(t,j=5.6,1h),4.90(dt,j=11.3,4.5,1h),4.61–4.50(m,1h),3.27(dp,j=20.4,6.7,6h),3.04(s,2h),2.97(d,j=11.4,2h),2.92–2.65(m,5h),2.32(dd,j=11.6,9.8,2h),2.15(s,4h),1.83–1.49(m,11h),1.39–1.29(m,22h).13cnmr(101mhz,cdcl3)δ=171.20,170.41,169.55,168.62,167.66,157.49,155.38,155.23,146.99,144.89,138.49,137.43,136.07,134.62,132.55,131.28,127.75,127.16,124.88,123.62,123.10,121.14,116.59,111.35,111.05,109.95,105.77,71.65,62.06,60.39,55.48,55.00,48.93,42.56,38.91,37.59,33.03,31.48,29.72,29.23,29.18,29.15,26.90,26.83,22.81,22.18,21.04,18.92,15.37,14.20.hrms(dart-tof)calculatedforc51h65cln9o8s[m+h]+m/z998.4365,found998.4349.3、b系列protac分子的结构与合成中间体b1-m的合成:在20mlnmp中,加入3-氟-n-(2,6-二氧代-3-哌啶基)邻苯二甲酰亚胺(128mg,0.5mmol),[2-(2-氨基乙氧基)乙基]氨基甲酸叔丁酯(102mg,0.5mmol),dipea(129mg,1mmol),90℃下反应10h,tlc监测反应。反应完成后,在反应液中加入150mlea混匀后,首先用10%柠檬酸溶液洗2次,每次20ml,后用饱和nahco3溶液洗2次,每次20ml,最后用饱和食盐水洗2次后用无水硫酸钠干燥,减压浓缩后用pe/ea分离体系进行柱层析分离。产物为黄色固体,产率约为69%。1hnmr(400mhz,cdcl3)δ=8.27(s,1h),7.57–7.45(m,1h),7.11(d,j=7.1,1h),6.95(t,j=17.6,1h),6.52(s,1h),5.16–4.83(m,2h),3.81–3.23(m,8h),3.03–2.58(m,3h),2.14(dd,j=23.0,16.2,1h),1.44(d,j=9.7,9h).hrms(dart-tof)calculatedforc22h28n4o7na[m+na]+m/z483.1856,found483.1860.中间体b2-m的合成:在20mlnmp中,加入2-(2-(2-氨基乙氧基)乙氧基)乙基氨基甲酸叔丁酯(124mg,0.5mmol),其他原料和操作方法同b1-m。得黄色粉末,产率约为64%。1hnmr(400mhz,meod)δ=7.44(ddd,j=8.5,7.9,4.4,1h),7.04–6.89(m,2h),4.95(dd,j=12.4,5.3,1h),3.62(dd,j=7.1,3.2,2h),3.59–3.47(m,4h),3.41(t,j=5.6,4h),3.21(dt,j=3.2,1.6,1h),3.11(t,j=5.6,2h),2.84–2.53(m,3h),2.07–1.95(m,1h),1.27(d,j=29.5,9h).hrms(dart-tof)calculatedforc24h33n4o8[m+h]+m/z505.2298,found505.2291.中间体b3-m的合成:在20mlnmp中,加入13-氨基-5,8,11-三氧杂-2-氮杂十三烷酸1,1-二甲基乙酯(146mg,0.5mmol),其他原料和操作方法同b1-m。得黄色粉末,产率约为61%。1hnmr(400mhz,meod)δ=7.44(dt,j=14.2,5.5,1h),7.04–6.92(m,2h),4.95(dd,j=12.6,5.4,1h),3.62(t,j=4.9,2h),3.56(s,4h),3.50(ddd,j=9.2,6.5,3.9,4h),3.44–3.35(m,4h),3.27–3.17(m,2h),3.08(dd,j=13.2,7.7,2h),2.83–2.54(m,3h),2.08–1.95(m,1h),1.32(s,9h).hrms(dart-tof)calculatedforc26h37n4o9[m+h]+m/z549.2561,found549.2553.中间体b4-m的合成:在20mlnmp中,加入16-氨基-5,8,11,14-四氧杂-2-氮杂十六烷酸1,1-二甲基乙酯(170mg,0.5mmol),其他原料和操作方法同b1-m。得黄色粉末,产率约为63%。1hnmr(400mhz,cdcl3)δ=7.49(dd,j=8.3,7.4,1h),7.10(d,j=7.0,1h),6.92(d,j=8.5,1h),4.91(dd,j=12.1,5.3,1h),3.72(t,j=5.4,2h),3.70–3.58(m,10h),3.54(t,j=5.1,2h),3.47(q,j=5.5,2h),3.30(d,j=4.8,2h),2.94–2.66(m,3h),2.17–2.07(m,1h),1.44(s,9h).13cnmr(101mhz,cdcl3)δ=171.03,169.26,168.34,167.60,156.04,146.88,136.04,132.55,116.79,111.67,110.35,70.80,70.67,70.62,70.47,70.27,69.49,48.88,42.42,31.42,29.70,28.44,22.83,14.11.hrms(dart-tof)calculatedforc28h41n4o10s[m+h]+m/z593.2823,found593.2815.目标产物b1的合成:将b1-m(92mg,0.2mmol)溶于5ml三氟乙酸中,常温搅拌5h。停止反应,在反应液中加入10mldcm并减压浓缩,得棕色油状物。在该油状物中加入10mldmf溶解,加入中间体2(63mg,0.1mmol),hatu(45.6mg,0.12mmol),dipea(38.7mg,0.3mmol)。常温反应15h,tlc监测反应进度。反应完成后用30ml水淬灭反应,用ea萃取水层4次,合并有机层,用饱和食盐水反洗2次,用无水硫酸钠干燥,减压浓缩,用dcm/meoh分离体系进行柱层析分离。产物为黄色粉末,产率约46%.1hnmr(400mhz,cdcl3)δ=9.49(s,1h),8.57(d,j=8.3,1h),8.15(s,1h),7.97(s,1h),7.92(dd,j=7.9,1.3,1h),7.67–7.58(m,1h),7.54(s,1h),7.53–7.47(m,1h),7.24(d,j=7.8,1h),7.13(d,j=7.1,1h),6.86(d,j=8.5,1h),6.77(s,1h),4.89(dd,j=11.9,5.6,1h),4.61–4.46(m,1h),3.80–3.21(m,12h),2.90–2.45(m,9h),2.24–2.10(m,5h),1.78(d,j=13.2,3h),1.37–1.30(m,13h).13cnmr(101mhz,cdcl3)δ=172.53,171.83,171.14,170.11,167.47,157.51,155.33,146.75,144.73,138.48,136.32,134.67,132.51,131.24,127.70,127.15,124.88,123.71,123.13,120.76,116.79,111.92,111.32,110.49,105.69,71.81,70.16,68.31,60.39,55.46,54.54,53.88,53.22,48.91,41.67,39.17,37.72,32.56,31.74,31.48,29.69,23.65,22.27,22.26,21.04,18.93,15.37,14.20.hrms(dart-tof)calculatedforc48h59cln9o9s[m+h]+m/z972.3845,found972.3887.目标产物b2的合成:称取原料b2-m(250mg,0.5mmol),其它原料和操作方法同目标产物b1。得目标产物为黄色粉末,产率约为41%。1hnmr(400mhz,cdcl3)δ=9.51(s,1h),8.57(d,j=8.3,1h),8.15(s,1h),8.00(d,j=11.6,1h),7.92(d,j=7.9,1h),7.65–7.54(m,3h),7.47(t,j=7.8,1h),7.27–7.21(m,1h),7.08(d,j=7.0,1h),6.88(t,j=8.8,1h),6.75(d,j=5.0,1h),6.52(t,j=5.1,1h),4.92(dd,j=11.9,5.7,1h),4.55(dt,j=12.0,6.0,1h),3.77–3.57(m,7h),3.51–3.39(m,4h),3.37–3.29(m,2h),2.98(s,2h),2.79(ddd,j=29.5,19.9,8.9,5h),2.60–2.42(m,4h),2.18–2.11(m,4h),1.84(s,4h),1.33(dd,j=11.2,6.4,11h).13cnmr(101mhz,cdcl3)δ=172.26,172.05,169.54,169.45,167.62,157.45,155.35,155.29,146.73,144.80,138.46,136.17,134.68,132.52,131.28,127.96,127.18,124.85,123.59,123.19,120.88,116.82,111.73,110.95,110.30,71.74,70.70,70.03,69.83,69.12,60.40,55.50,53.82,48.91,42.20,39.44,31.38,22.95,22.21,21.05,18.92,15.37,14.20.hrms(dart-tof)calculatedforc50h63cln9o10s[m+h]+m/z1016.4107,found1016.4116.目标产物b3的合成:称取原料b3-m(110mg,0.2mmol),其它原料和操作方法同目标产物b1。得目标产物为黄色粉末,产率约为45%。1hnmr(400mhz,cdcl3)δ=9.51(s,1h),8.57(d,j=8.4,1h),8.16(s,1h),7.99(d,j=13.0,1h),7.92(d,j=7.9,1h),7.83(s,1h),7.67–7.54(m,2h),7.46(t,j=7.7,1h),7.27–7.20(m,1h),7.06(d,j=7.0,1h),6.89(d,j=8.6,1h),6.77(s,1h),6.46(d,j=5.1,1h),4.93(dd,j=11.7,5.7,1h),4.56(dt,j=12.0,6.0,1h),3.78–3.52(m,12h),3.45(d,j=4.7,4h),3.25(dd,j=13.4,6.7,3h),2.95–2.65(m,7h),2.59–2.33(m,4h),2.17–2.11(m,3h),1.80(d,j=21.0,4h),1.41–1.28(m,12h).13cnmr(101mhz,cdcl3)δ=172.74,172.07,171.16,169.44,169.41,167.65,157.46,155.34,155.27,146.75,144.83,138.46,136.14,134.69,132.45,131.26,127.85,127.12,124.81,123.59,123.17,120.93,116.89,111.67,110.96,110.26,105.79,71.69,70.43,70.27,70.07,69.96,69.36,60.39,55.50,54.08,53.79,48.90,42.22,39.14,37.22,31.81,31.39,22.75,22.19,21.04,18.91,15.36,14.20.hrms(dart-tof)calculatedforc52h67cln9o11s[m+h]+m/z1060.4369,found1060.4349.目标产物b4的合成:称取原料b4-m(120mg,0.2mmol),其它原料和操作方法同目标产物b1。得目标产物为黄色粉末,产率约为47%。1hnmr(400mhz,meod)δ=8.35(d,j=8.5,1h),8.05(d,j=1.5,1h),7.82(d,j=8.0,1h),7.66(t,j=6.0,1h),7.60–7.54(m,1h),7.43(ddd,j=9.6,8.5,6.0,1h),7.26(t,j=7.7,1h),7.03–6.88(m,2h),6.74(d,j=3.8,1h),6.20–6.08(m,1h),5.00–4.90(m,1h),4.56–4.43(m,1h),3.66–3.25(m,15h),2.92–2.33(m,8h),2.09–1.99(m,3h),1.27–1.14(m,17h).hrms(dart-tof)calculatedforc54h71cln9o12s[m+h]+m/z1104.4631,found1104.4629.目标产物b5的合成:该目标产物的制备方法同b1,原料为中间体4和b1-m,其他原料和处理方法相同。产率为35%1hnmr(400mhz,cdcl3)δ=9.51(s,1h),8.57(t,j=6.7,1h),8.16(s,1h),8.01(d,j=6.1,1h),7.93(dd,j=8.0,1.4,1h),7.66–7.59(m,1h),7.54(s,1h),7.51–7.45(m,1h),7.42(t,j=5.8,1h),7.25(d,j=7.5,1h),7.10(d,j=7.1,1h),6.93–6.86(m,1h),6.49(t,j=5.6,1h),4.92–4.83(m,1h),4.54(dq,j=12.1,6.0,1h),3.80–3.43(m,9h),3.26(dt,j=13.7,6.8,1h),3.07(s,2h),2.98(d,j=11.4,2h),2.87–2.63(m,4h),2.32(t,j=11.0,2h),2.15(s,3h),2.10–2.02(m,2h),1.72(d,j=15.4,5h),1.36–1.30(m,12h).13cnmr(101mhz,cdcl3)δ=170.91,170.79,169.40,168.28,167.49,157.48,155.36,146.73,144.73,138.50,137.39,136.17,134.64,132.52,131.29,127.79,127.25,124.91,123.63,123.12,120.85,116.63,111.90,111.14,110.47,105.86,71.76,70.11,69.28,61.96,55.47,54.85,48.91,42.38,38.75,37.65,32.94,31.37,29.70,22.76,22.25,18.92,15.37.hrms(dart-tof)calculatedforc47h57cln9o9s[m+h]+m/z958.3688,found958.3676.目标产物b6的合成:该目标产物的制备方法同b1,原料为中间体4和b2-m,其他原料和处理方法相同。产率为31%1hnmr(400mhz,cdcl3)δ=9.53(s,1h),8.58(d,j=8.2,1h),8.16(s,1h),7.97(s,1h),7.93(dd,j=8.0,1.6,1h),7.60(dd,j=11.3,4.2,2h),7.49(ddd,j=9.5,8.3,4.3,2h),7.27–7.21(m,2h),7.10(d,j=7.1,1h),6.87(d,j=8.5,1h),6.78(s,1h),6.50(t,j=5.4,1h),4.89(dt,j=11.4,5.8,1h),4.63–4.52(m,1h),3.78–3.61(m,10h),3.57–3.42(m,5h),3.31–3.23(m,1h),3.08(d,j=16.4,2h),2.98(d,j=8.6,2h),2.90–2.59(m,6h),2.30(t,j=10.4,3h),2.13(d,j=6.9,4h),1.70(dd,j=20.5,10.9,5h),1.39–1.29(m,14h).13cnmr(101mhz,cdcl3)δ=171.27,170.76,169.36,168.74,167.59,157.50,155.38,146.73,144.83,138.50,137.53,136.04,134.63,132.61,131.28,127.68,127.10,124.88,123.11,120.99,116.62,111.73,111.05,110.49,105.78,99.99,71.59,70.81,70.27,70.16,69.37,55.48,54.90,48.95,42.29,37.57,32.93,31.43,22.93,22.20,18.92,15.37.hrms(dart-tof)calculatedforc49h61cln9o10s[m+h]+m/z1002.3951,found958.3676.目标产物b7的合成:该目标产物的制备方法同b1,原料为中间体4和b3-m,其他原料和处理方法相同。产率为27%1hnmr(400mhz,cdcl3)δ=9.52(s,1h),8.58(d,j=7.9,1h),8.18(s,1h),7.97(s,1h),7.92(dd,j=8.0,1.6,1h),7.67(s,1h),7.63–7.57(m,1h),7.47(dd,j=8.3,7.3,1h),7.27–7.22(m,2h),7.09(d,j=6.9,1h),6.89(d,j=8.5,1h),6.78(s,1h),6.46(t,j=5.6,1h),4.92(dt,j=9.7,7.1,1h),4.64–4.53(m,1h),3.73–3.63(m,10h),3.59(t,j=5.1,2h),3.51(dd,j=10.5,5.2,2h),3.44(dd,j=10.8,5.4,2h),3.25(dq,j=13.7,6.9,1h),3.08(d,j=14.6,2h),2.97(d,j=11.0,2h),2.91–2.63(m,4h),2.30(t,j=10.0,2h),2.14(s,3h),1.73(dd,j=20.5,12.0,4h),1.37–1.30(m,13h).13cnmr(101mhz,cdcl3)δ=171.36,169.32,168.70,167.64,157.50,155.38,155.22,146.78,144.91,138.49,137.54,135.99,134.64,132.57,131.27,127.62,126.98,124.86,123.62,123.10,121.10,116.68,111.65,110.94,110.38,105.74,71.52,70.83,70.62,70.51,70.34,70.13,69.51,62.06,60.39,55.48,54.95,48.94,42.41,38.79,37.59,32.96,31.51,29.69,22.80,22.17,21.05,18.92,15.37,14.20.hrms(dart-tof)calculatedforc51h65cln9o11s[m+h]+m/z1046.4213,found1046.4213.4、c系列protac分子的结构与合成中间体c-m的合成:称取泊马度胺(546mg,2mmol),溶于100mlthf中,加入氯乙酰氯(452mg,4mmol),回流5h,用tlc监测反应。先减压浓缩,除去thf,用200ml的ea溶解,后用水反洗3次。有机层用无水硫酸钠干燥,减压浓缩,用pe/ea作为流动相进行柱层析分离。得白色固体,产率约为70%。1hnmr(400mhz,cdcl3)δ=8.78(t,j=12.7,1h),7.80–7.70(m,1h),7.60(d,j=7.3,1h),4.97(dd,j=12.4,5.3,1h),3.98–3.85(m,2h),3.02–2.68(m,3h),2.20(ddd,j=12.4,10.1,6.3,1h).hrms(dart-tof)calculatedforc15h13cln3o5[m+h]+m/z350.0544,found350.0537.中间体c1-m的合成:称取中间体c-m(70mg,0.2mmol),溶于10mlthf中,加入n-叔丁氧羰基-1,4-丁二胺(113mg,0.6mmol),碳酸钾(83mg,0.6mmol),回流5h,用tlc监测反应。加入30ml水淬灭反应,用100ml的ea萃取3次,后用水反洗3次。有机层用无水硫酸钠干燥,减压浓缩,用pe/ea作为流动相进行柱层析分离。得黄色固体,产率约为70%。1hnmr(400mhz,cdcl3)δ=11.24(d,j=20.3,1h),8.90(d,j=8.4,1h),7.79–7.66(m,1h),7.55(t,j=10.5,1h),5.22–4.73(m,2h),3.20(dddd,j=94.9,67.2,44.2,26.5,9h),2.18(s,1h),1.65–1.36(m,12h).hrms(dart-tof)calculatedforc24h32n5o7[m+h]+m/z502.2302,found502.2293.中间体c2-m的合成:该中间体的制备方法同c1-m,原料为n-叔丁氧羰基-1,6-己二胺,其他原料和处理方法相同。产率为65%1hnmr(400mhz,cdcl3)δ=8.91(d,j=8.5,1h),7.75–7.68(m,1h),7.57(t,j=5.8,1h),4.99(dd,j=12.2,5.2,1h),3.46(dd,j=33.9,17.4,2h),3.24–2.56(m,7h),2.28–2.10(m,1h),1.65(dd,j=52.8,47.1,4h),1.44(s,10h),1.39–1.19(m,8h).hrms(dart-tof)calculatedforc26h36n5o7[m+h]+m/z530.2615,found530.2606.中间体c3-m的合成:该中间体的制备方法同c1-m,原料为n-叔丁氧羰基-1,8-辛二胺,其他原料和处理方法相同。产率为61%1hnmr(400mhz,cdcl3)δ=8.96–8.73(m,1h),7.73(dt,j=11.9,7.9,1h),7.63–7.52(m,1h),4.98(dt,j=12.5,11.2,1h),3.56–3.44(m,1h),3.11(t,j=17.3,2h),2.96–2.61(m,4h),2.16(dt,j=10.2,6.2,1h),1.91(d,j=47.5,2h),1.57(dd,j=13.9,6.9,1h),1.44(s,9h),1.38–1.20(m,10h).hrms(dart-tof)calculatedforc28h40n5o7[m+h]+m/z558.2928,found558.2921.目标产物c1的合成:将c1-m(92mg,0.2mmol)溶于5ml三氟乙酸中,50℃下搅拌1h。停止反应,在反应液中加入10mldcm并减压浓缩,得棕色油状物。在该油状物中加入10mldmf溶解,加入中间体2(63mg,0.1mmol),hatu(45.6mg,0.12mmol),dipea(38.7mg,0.3mmol)。常温反应15h,tlc监测反应进度。反应完成后用30ml水淬灭反应,用ea萃取水层4次,合并有机层,用饱和食盐水反洗2次,用无水硫酸钠干燥,减压浓缩,用dcm/meoh分离体系进行柱层析分离。产物为黄色粉末,产率约46%.1hnmr(400mhz,cdcl3)δ=9.51(s,1h),8.89(d,j=8.4,0h),8.57(d,j=8.3,1h),8.16(s,1h),8.01(d,j=4.5,1h),7.93(d,j=7.8,1h),7.75–7.65(m,1h),7.65–7.59(m,1h),7.55(d,j=6.7,1h),7.25(d,j=9.3,3h),6.80–6.72(m,1h),5.06–4.86(m,1h),4.55(dd,j=12.0,5.9,1h),3.30(ddd,j=20.4,15.4,7.6,6h),3.00–2.61(m,7h),2.21–2.13(m,3h),1.97–1.49(m,11h),1.38–1.29(m,13h).hrms(dart-tof)calculatedforc50h62cln10o9s[m+h]+m/z1013.4110,found1013.4182.目标产物c2的合成:该目标产物的制备方法同c1,原料为c2-m,其他原料和处理方法相同。产率为45%1hnmr(400mhz,cdcl3)δ=11.12(s,1h),9.44(s,1h),8.83(d,j=8.4,1h),8.50(d,j=8.2,1h),8.09(s,1h),7.92(s,1h),7.86(d,j=7.8,1h),7.63(t,j=7.7,1h),7.58–7.44(m,3h),7.18(d,j=11.8,2h),6.70(s,1h),4.90(dd,j=11.6,5.1,1h),4.47(dt,j=11.7,5.8,1h),3.35(q,j=17.6,2h),3.27–3.06(m,5h),2.95–2.51(m,10h),2.43(s,2h),2.19(s,3h),2.07(s,3h),1.74(s,4h),1.47(ddd,j=22.2,16.0,7.8,6h),1.26(dd,j=13.5,6.4,12h).13cnmr(101mhz,cdcl3)δ=172.01,171.73,168.63,168.33,166.98,157.45,155.29,144.78,138.48,137.08,136.13,134.63,131.52,131.28,127.88,127.11,125.16,124.94,123.63,123.13,120.87,118.43,116.02,111.01,105.86,100.00,71.74,60.40,55.47,54.10,53.81,53.53,50.44,49.27,39.24,37.59,32.42,31.57,29.96,29.54,26.96,26.77,22.74,22.22,18.92,15.37,14.20.hrms(dart-tof)calculatedforc52h66cln10o9s[m+h]+m/z1041.4423,found1041.4438.目标产物c3的合成:该目标产物的制备方法同c1,原料为c3-m,其他原料和处理方法相同。产率为48%。1hnmr(400mhz,cdcl3)δ=9.52(s,1h),8.58(d,j=8.3,1h),8.17(d,j=6.8,1h),8.17(d,j=6.8,1h),8.01(d,j=9.4,1h),7.93(d,j=7.9,1h),7.71(dt,j=12.6,6.4,1h),7.66–7.50(m,3h),7.24(d,j=7.7,1h),4.96(dd,j=12.0,5.1,1h),4.52(dd,j=12.0,5.9,1h),3.57–3.38(m,2h),3.33–3.13(m,5h),2.95–2.40(m,10h),2.28(s,2h),2.15(s,3h),1.96(d,j=6.3,2h),1.90–1.70(m,5h),1.32(ddd,j=26.8,14.8,6.7,27h).hrms(dart-tof)calculatedforc54h70cln10o9s[m+h]+m/z1069.4736,found1069.4791.5、d系列protac分子的结构与合成:中间体d1-m的合成:该中间体的制备方法同c1-m,原料为[2-(2-氨基乙氧基)乙基]氨基甲酸叔丁酯,其它原料和处理方法相同。产率为61%1hnmr(400mhz,cdcl3)δ=11.19(s,1h),8.90(d,j=8.5,1h),7.76–7.67(m,1h),7.56(d,j=7.2,1h),4.99(s,2h),3.65(dd,j=12.5,7.6,2h),3.53(dd,j=11.4,6.1,4h),3.32(d,j=4.8,2h),2.96–2.74(m,5h),2.26–2.11(m,2h),1.41(d,j=26.9,9h).13cnmr(101mhz,cdcl3)δ=171.79,171.11,168.44,166.92,156.10,137.06,136.24,131.44,125.22,118.52,116.04,77.37,77.05,76.73,70.19,70.12,60.40,53.18,49.50,49.25,40.36,31.39,29.68,28.41,22.69,21.04,14.19.hrms(dart-tof)calculatedforc24h31n5o8na[m+na]+m/z540.2070,found540.2117.中间体d2-m的合成:该中间体的制备方法同c1-m,原料为2-(2-(2-氨基乙氧基)乙氧基)乙基氨基甲酸叔丁酯,其它原料和处理方法相同,产率为67%。1hnmr(400mhz,cdcl3)δ=11.21(s,1h),8.90(d,j=8.5,1h),7.71(t,j=7.9,1h),7.54(t,j=10.0,1h),4.98(dd,j=11.8,5.3,1h),3.71(dd,j=11.9,7.2,2h),3.63(s,4h),3.53(dd,j=10.2,5.2,2h),3.48(d,j=17.6,2h),2.97–2.69(m,5h),2.15(dd,j=16.4,11.8,3h),1.43(s,10h).13cnmr(101mhz,cdcl3)δ=171.87,171.17,168.37,166.92,156.06,137.10,136.22,131.43,125.23,118.48,116.02,79.31,77.37,77.05,76.74,70.38,70.28,70.24,70.13,60.39,53.06,49.42,49.23,40.35,31.40,29.68,28.41,22.67,21.03,14.19.hrms(dart-tof)calculatedforc26h36n5o9[m+h]+m/z562.2513,found562.2510.中间体d3-m的合成:该中间体的制备方法同c1-m,原料为13-氨基-5,8,11-三氧杂-2-氮杂十三烷酸1,1-二甲基乙酯,其它原料和处理方法相同,产率为59%。1hnmr(400mhz,cdcl3)δ=11.15(s,1h),8.78(dd,j=15.0,8.2,1h),7.66–7.60(m,1h),7.46(d,j=7.3,1h),4.92(dt,j=9.4,8.7,1h),3.81–3.32(m,16h),3.22(d,j=4.5,2h),2.89–2.63(m,5h),2.39(s,2h),1.35(s,9h).13cnmr(101mhz,cdcl3)δ=172.01,171.57,171.13,168.44,168.31,166.93,156.03,137.02,136.13,131.40,125.09,118.37,115.98,79.10,70.39,70.37,70.30,70.22,70.19,60.34,52.99,49.34,49.20,40.28,31.36,29.61,28.38,22.61,20.98,14.15.hrms(dart-tof)calculatedforc28h40n5o10[m+h]+m/z606.2775,found606.2772.目标产物d1的合成:该目标产物制备方法同c1,原料为d1-m,其它原料和处理方法相同,产率为47%。1hnmr(400mhz,cdcl3)δ=11.18(s,1h),9.51(s,1h),8.87(d,j=8.5,1h),8.58(d,j=8.3,1h),8.14(d,j=11.3,1h),7.99(s,1h),7.92(dd,j=7.9,1.3,1h),7.75–7.66(m,1h),7.65–7.58(m,1h),7.58–7.50(m,2h),7.25(d,j=7.6,1h),6.77(s,1h),4.96(dd,j=12.1,5.4,1h),4.60–4.48(m,1h),3.78–3.53(m,4h),3.53–3.38(m,4h),3.34–3.07(m,4h),2.96–2.61(m,9h),2.47(t,j=6.4,2h),2.15(s,3h),1.88–1.61(m,5h),1.38–1.30(m,13h).13cnmr(101mhz,cdcl3)δ=172.72,171.80,171.45,168.55,168.50,166.89,157.47,155.34,144.76,138.49,137.02,136.23,134.65,131.47,131.27,127.85,127.25,125.14,124.87,123.62,123.12,120.87,118.53,116.03,111.18,105.83,71.83,70.23,69.83,60.40,55.47,54.12,53.99,53.83,53.30,49.57,49.28,39.02,37.83,32.45,31.42,29.69,22.83,22.69,22.26,21.04,18.92,15.37,14.20,14.12.hrms(dart-tof)calculatedforc50h60cln10o10s[m+h]+m/z1029.4060,found1029.4071.目标产物d2的合成:该目标产物制备方法同c1,原料为d2-m,其它原料和处理方法相同,产率为51%。1hnmr(400mhz,cdcl3)δ=11.21(s,1h),9.50(s,1h),8.88(d,j=8.5,1h),8.57(d,j=8.4,1h),8.14(s,1h),7.97(s,1h),7.92(d,j=7.5,2h),7.75–7.66(m,1h),7.66–7.57(m,2h),7.55(d,j=7.2,1h),7.24(d,j=7.6,1h),6.78(s,1h),4.96(dd,j=12.1,5.3,1h),4.54(dt,j=12.1,6.0,1h),3.77–3.38(m,14h),3.26(dt,j=13.7,6.8,1h),3.13(d,j=11.2,2h),2.97–2.61(m,10h),2.45(t,j=6.5,2h),2.15(d,j=6.4,7h),1.75(d,j=11.4,5h),1.38–1.29(m,14h).13cnmr(101mhz,cdcl3)δ=172.51,171.91,171.40,168.49,168.45,166.92,157.47,155.34,155.26,144.80,138.48,137.41,137.08,136.19,134.66,131.45,131.26,127.70,127.09,125.15,124.86,123.63,123.11,120.88,118.45,116.01,111.12,105.76,71.67,70.55,70.23,70.06,69.97,55.47,54.20,53.91,53.18,49.40,49.30,38.97,37.95,32.85,32.52,31.52,29.69,22.71,22.22,18.93,15.36,14.20.hrms(dart-tof)calculatedforc52h66cln10o11s[m+h]+m/z1073.4322,found1073.4313.目标产物d3的合成:该目标产物制备方法同c1,原料为d3-m,其它原料和处理方法相同,产率为49%。1hnmr(400mhz,cdcl3)δ=9.51(s,1h),8.77(d,j=7.7,1h),8.56(d,j=8.2,1h),8.15(s,1h),7.99(s,1h),7.92(d,j=8.1,1h),7.68(d,j=7.3,1h),7.65–7.59(m,1h),7.55(d,j=11.0,1h),7.52(s,1h),7.29–7.21(m,4h),6.74(s,1h),4.63–4.51(m,1h),3.64(s,10h),3.53(s,2h),3.39(d,j=6.8,4h),3.05(s,2h),2.84(s,6h),2.57(s,4h),2.13(s,5h),1.87(s,7h),1.32(dd,j=11.0,6.4,12h).hrms(dart-tof)calculatedforc54h70cln10o12s[m+h]+m/z1117.4584,found1117.4648.6、e系列protac分子的结构及合成:中间体e-m的制备:称取泊马度胺(546mg,2mmol),溶于100mlthf中,加入丙烯酰氯(362mg,4mmol),回流5h,用tlc监测反应。先减压浓缩,除去thf,用200ml的ea溶解,后用水反洗3次。有机层用无水硫酸钠干燥,减压浓缩,用pe/ea作为流动相进行柱层析分离。得黄色固体,产率约为70%。1hnmr(400mhz,cdcl3)δ=9.54(d,j=37.5,1h),8.91(d,j=8.5,1h),8.03(s,1h),7.82–7.69(m,1h),7.58(d,j=7.2,1h),6.48(d,j=17.0,1h),6.31(dd,j=17.0,10.3,1h),5.89(d,j=10.3,1h),4.96(dd,j=12.3,5.4,1h),2.84(dddd,j=36.3,29.1,14.4,3.6,3h),2.27–2.13(m,1h).hrms(dart-tof)calculatedforc16h14n3o5[m+h]+m/z328.0928,found328.0919.中间体e1-m的合成:称取e-m(65mg,0.2mmol),溶于10ml的thf中,加入n-叔丁氧羰基-1,4-丁二胺(56.5mg,0.3mmol),三乙胺(60mg,0.6mmol)。40℃下反应12小时,tlc监测反应。反应完全后,加入30ml水淬灭反应,用ea萃取水层4次,合并有机层,用饱和食盐水反洗2次,用无水硫酸钠干燥,减压浓缩,用dcm/meoh分离体系进行柱层析分离。产物为黄色粉末,产率约52%.1hnmr(400mhz,cdcl3)δ=8.76(dd,j=16.2,8.5,1h),7.74–7.65(m,1h),7.56(t,j=8.7,1h),4.97(s,1h),3.28–2.51(m,13h),1.53–1.36(m,13h).hrms(dart-tof)calculatedforc25h34n5o7[m+h]+m/z516.2750,found516.2460.中间体e2-m的合成:该中间体的制备方法同e1-m,原料为n-叔丁氧羰基-1,6-己二胺,其他原料和处理方法相同。产率为48%1hnmr(400mhz,cdcl3)δ=8.77(d,j=8.4,1h),7.69(t,j=7.9,1h),7.54(d,j=7.3,1h),5.00–4.92(m,1h),3.24–2.60(m,13h),1.39(d,j=41.4,19h).hrms(dart-tof)calculatedforc27h38n5o7[m+h]+m/z544.2771,found544.2775.中间体e3-m的合成:该中间体的制备方法同e1-m,原料为n-叔丁氧羰基-1,8-辛二胺,其他原料和处理方法相同。产率为48%1hnmr(400mhz,cdcl3)δ=8.70(t,j=9.7,1h),7.63(dd,j=17.9,10.4,1h),7.48(t,j=9.5,1h),3.14–2.56(m,13h),2.12–2.02(m,1h),1.42–1.19(m,23h).hrms(dart-tof)calculatedforc29h42n5o7[m+h]+m/z572.3084,found572.3110.目标产物e1的合成:将e1-m(103mg,0.2mmol)溶于5ml三氟乙酸中,50℃下搅拌1h。停止反应,在反应液中加入10mldcm并减压浓缩,得棕色油状物。在该油状物中加入10mldmf溶解,加入中间体2(63mg,0.1mmol),hatu(45.6mg,0.12mmol),dipea(38.7mg,0.3mmol)。常温反应15h,tlc监测反应进度。反应完成后用30ml水淬灭反应,用ea萃取水层4次,合并有机层,用饱和食盐水反洗2次,用无水硫酸钠干燥,减压浓缩,用dcm/meoh分离体系进行柱层析分离。产物为黄色粉末,产率约46%.1hnmr(400mhz,cdcl3)δ=9.51(s,1h),8.81–8.65(m,1h),8.56(t,j=9.4,1h),8.20–8.10(m,1h),8.08–7.98(m,1h),7.93(d,j=7.5,1h),7.71(d,j=5.2,1h),7.67–7.48(m,3h),7.24(s,1h),6.80(d,j=10.0,1h),4.96(s,1h),4.57(s,1h),3.77(dd,j=22.6,16.6,2h),3.60–3.12(m,7h),2.81(dd,j=91.0,52.5,8h),2.14(d,j=4.9,3h),2.09–1.52(m,14h),1.40–1.28(m,16h).hrms(dart-tof)calculatedforc51h64cln10o9s[m+h]+m/z1027.4267,found1027.4261.目标产物e2的合成:该目标产物制备方法同e1,原料为e1-m,其它原料和处理方法相同,产率为31%。1hnmr(400mhz,cdcl3)δ=9.52(d,j=6.6,1h),8.57(t,j=8.6,1h),8.16(d,j=1.8,1h),8.05(d,j=13.6,1h),7.93(dd,j=8.0,1.5,1h),7.62(t,j=7.9,1h),7.56(d,j=4.2,1h),7.36–7.25(m,4h),6.74(d,j=8.0,1h),4.60(td,j=12.5,6.3,1h),4.05–3.95(m,1h),3.77(d,j=13.4,1h),3.54(s,1h),3.34–2.89(m,5h),2.84–2.67(m,1h),2.20(dd,j=24.5,6.7,3h),2.10(s,1h),1.94–1.49(m,8h),1.40–1.21(m,24h).hrms(dart-tof)calculatedforc53h68cln10o9s[m+h]+m/z1055.4580,found1055.4595.7、f系列protac分子的结构及合成中间体f1-m的合成:该中间体的制备方法同e1-m,原料为[2-(2-氨基乙氧基)乙基]氨基甲酸叔丁酯,其他原料和处理方法相同,产率为53%1hnmr(400mhz,cdcl3)δ=8.80(t,j=8.4,1h),7.69(dd,j=8.5,7.4,1h),7.55(d,j=7.2,1h),4.96(d,j=6.1,1h),3.69–3.59(m,2h),3.53(t,j=5.3,2h),3.31(d,j=4.2,2h),3.06(t,j=5.9,2h),2.96–2.85(m,3h),2.82–2.73(m,2h),2.68(t,j=6.0,2h),2.22–2.08(m,1h),1.43(d,j=6.8,10h).hrms(dart-tof)calculatedforc25h34n5o8[m+h]+m/z532.2407,found532.2399.中间体f2-m的合成:该中间体的制备方法同e1-m,原料为2-(2-(2-氨基乙氧基)乙氧基)乙基氨基甲酸叔丁酯,其他原料和处理方法相同,产率为41%。1hnmr(400mhz,cdcl3)δ=8.77(t,j=12.7,1h),7.68(dt,j=22.9,11.4,1h),7.53(t,j=9.2,1h),5.20(s,1h),5.01–4.90(m,1h),3.68(dd,j=10.0,5.1,2h),3.64(d,j=8.6,4h),3.54(t,j=5.1,2h),3.31(d,j=4.3,2h),3.09(t,j=5.8,2h),3.00–2.67(m,7h),2.21–2.08(m,1h),1.43(s,9h).hrms(dart-tof)calculatedforc27h38n5o9[m+h]+m/z576.2670,found576.2665.目标产物f1的合成:该目标产物制备方法同e1,原料为f1-m,其它原料和处理方法相同,产率为39%。1hnmr(400mhz,cdcl3)δ=9.52(s,1h),8.79(d,j=8.2,1h),8.58(d,j=8.0,1h),8.16(s,1h),8.05–7.97(m,1h),7.93(dd,j=8.0,1.5,1h),7.70–7.64(m,1h),7.64–7.58(m,1h),7.57(s,1h),7.53(d,j=6.8,1h),7.26–7.23(m,1h),6.77(s,1h),4.94(dd,j=12.4,5.4,1h),4.54(dt,j=12.1,6.1,1h),3.71–3.54(m,4h),3.49–3.43(m,2h),3.29–3.18(m,2h),3.04(t,j=5.9,2h),2.78(dddd,j=21.3,18.4,10.7,5.0,9h),2.53(t,j=6.2,2h),2.14(s,3h),1.80(s,3h),1.33(dd,j=11.8,6.4,10h).13cnmr(101mhz,cdcl3)δ=172.27,171.65,171.41,168.53,168.53,168.26,166.84,157.45,155.35,144.80,138.49,137.33,136.04,134.64,134.64,131.48,131.28,127.94,127.94,127.12,126.18,124.89,123.60,123.13,120.85,118.47,116.18,111.07,105.88,71.79,69.74,55.48,53.86,49.25,48.77,44.77,39.07,37.43,31.93,31.45,29.70,29.36,22.69,22.24,18.93,15.37,14.12.hrms(dart-tof)calculatedforc51h64cln10o10s[m+h]+m/z1043.4216,found1043.4178.目标产物f2的合成:该目标产物制备方法同e1,原料为f2-m,其它原料和处理方法相同,产率为31%。1hnmr(400mhz,cdcl3)δ=9.51(s,1h),8.82(d,j=8.0,1h),8.58(d,j=8.3,1h),8.17–8.13(m,1h),8.00(dd,j=8.6,4.2,1h),7.93(dd,j=8.0,1.5,1h),7.68(dd,j=8.5,7.3,1h),7.65–7.58(m,2h),7.53(d,j=6.7,1h),7.26–7.22(m,1h),6.78(d,j=4.4,1h),5.01–4.88(m,1h),4.55(dt,j=12.2,6.0,1h),3.79–3.36(m,13h),3.25(dd,j=13.1,6.2,1h),3.13(d,j=10.7,2h),3.04–2.98(m,1h),2.92–2.83(m,2h),2.82–2.69(m,4h),2.62–2.55(m,1h),2.46(t,j=6.5,2h),2.25–2.11(m,6h),1.76(d,j=17.4,8h),1.39–1.31(m,12h).13cnmr(101mhz,cdcl3)δ=172.05,171.32,168.47,168.21,166.85,157.49,155.35,144.80,138.51,137.49,137.31,136.04,134.64,131.28,127.73,127.16,127.07,126.27,124.88,123.64,123.10,118.39,116.15,111.12,71.68,70.20,70.13,70.00,55.47,53.99,48.74,44.83,38.95,37.93,37.30,32.88,32.47,31.93,29.70,29.36,22.69,22.23,18.94,15.37.hrms(dart-tof)calculatedforc53h68cln10o11s[m+h]+m/z1087.4478,found1087.4503.8、g系列的protac的结构与合成中间体g-m的合成:称取3-氨基-n-(2,6-二氧代-3-哌啶基)邻苯二甲酰亚胺(411mg,0.15mmol)溶于dmf中,加入溴乙酸叔丁酯(293mg,0.15mmol),碳酸钾(621mg,4.5mmol),常温搅拌15h,tlc监测反应,反应完全后在反应液中加入30ml水淬灭反应,用ea萃取水层4次,合并有机层,用饱和食盐水反洗2次,用无水硫酸钠干燥,减压浓缩,用pe/ea分离体系进行柱层析分离。产物为白色粉末,产率约72%.1hnmr(400mhz,cdcl3)δ=7.67(dd,j=8.4,7.4,1h),7.52(d,j=7.1,1h),7.11(d,j=8.4,1h),4.97(dd,j=12.2,5.3,1h),4.77(d,j=16.7,2h),3.02–2.63(m,4h),1.48(s,8h).hrms(dart-tof)calculatedforc19h21n2o7[m+h]+m/z389.1349,found389.1339.中间体g1-m的合成:将g-m(78mg,0.2mmol)溶于5ml三氟乙酸中,50℃下搅拌1h。停止反应,在反应液中加入10mldcm并减压浓缩,得白色固体,加入10mldmf溶解,加入n-叔丁氧羰基-1,4-丁二胺(54mg,0.3mmol),hatu(91mg,0.24mmol),dipea(38.7mg,0.3mmol)。常温反应15h,tlc监测反应进度。反应完成后用30ml水淬灭反应,用ea萃取水层4次,合并有机层,用饱和食盐水反洗2次,用无水硫酸钠干燥,减压浓缩,用dcm/meoh分离体系进行柱层析分离。产物为黄色粉末,产率约56%.1hnmr(400mhz,cdcl3)δ=7.76(dd,j=8.3,7.4,1h),7.57(d,j=7.3,1h),7.22(d,j=8.4,1h),5.01(d,j=6.0,1h),4.79–4.55(m,2h),3.56(s,1h),3.21(s,2h),2.99–2.70(m,3h),2.17(qd,j=5.6,2.9,1h),1.65–1.51(m,4h),1.44(d,j=15.5,8h).hrms(dart-tof)calculatedforc24h30n4o7k[m+h]+m/z525.1752,found525.1985.中间体g2-m的合成:该中间体的制备方法同g1-m,原料为n-叔丁氧羰基-1,6-己二胺,其他原料和处理方法相同。产率为68%1hnmr(400mhz,cdcl3)δ=7.75(dd,j=8.3,7.4,1h),7.55(d,j=7.1,1h),7.21(d,j=8.4,1h),4.64(s,3h),3.49–3.15(m,4h),2.97–2.72(m,4h),1.70–1.55(m,5h),1.30–1.22(m,11h).hrms(dart-tof)calculatedforc26h35n4o7[m+h]+m/z515.2506,found515.2501.中间体g3-m的合成:该中间体的制备方法同g1-m,原料为n-叔丁氧羰基-1,8-辛二胺,其他原料和处理方法相同。产率为57%1hnmr(400mhz,cdcl3)δ=7.74(dd,j=8.3,7.5,1h),7.55(d,j=7.2,1h),7.20(d,j=8.3,1h),5.05–4.93(m,1h),4.64(s,2h),3.36(dd,j=13.0,6.8,2h),3.27–3.16(m,1h),2.83(dddd,j=20.9,16.1,10.6,2.9,4h),2.16(dt,j=9.8,6.4,1h),1.44(s,13h),1.30(s,9h).hrms(dart-tof)calculatedforc28h39n4o8[m+h]+m/z559.2768,found559.2761.目标产物g1的合成:将g1-m(100mg,0.2mmol)溶于5ml三氟乙酸中,50℃下搅拌1h。停止反应,在反应液中加入10mldcm并减压浓缩,得棕色油状物。在该油状物中加入10mldmf溶解,加入中间体2(63mg,0.1mmol),hatu(45.6mg,0.12mmol),dipea(38.7mg,0.3mmol)。常温反应15h,tlc监测反应进度。反应完成后用30ml水淬灭反应,用ea萃取水层4次,合并有机层,用饱和食盐水反洗2次,用无水硫酸钠干燥,减压浓缩,用dcm/meoh分离体系进行柱层析分离。产物为黄色粉末,产率约39%.1hnmr(400mhz,cdcl3)δ=9.51(s,1h),8.58(d,j=8.0,1h),8.16(s,1h),8.00(s,1h),7.93(dd,j=8.0,1.5,1h),7.73(dd,j=8.3,7.4,1h),7.66–7.59(m,2h),7.55(s,2h),7.27–7.23(m,1h),7.19(d,j=8.3,1h),6.77(s,1h),5.05–4.93(m,1h),4.60(dt,j=12.2,10.2,3h),3.32(dddd,j=34.8,27.9,13.6,7.1,7h),2.98–2.64(m,6h),2.50(d,j=6.2,2h),2.14(s,3h),1.78(d,j=18.6,4h),1.70–1.53(m,4h),1.42–1.29(m,12h).13cnmr(101mhz,cdcl3)δ=171.49,168.82,166.81,166.59,166.45,157.48,155.34,154.76,144.74,138.50,137.12,134.65,133.51,131.27,127.85,127.10,124.89,123.63,123.12,120.79,120.33,118.41,117.63,111.03,105.83,71.80,68.66,60.39,55.48,53.92,53.78,49.44,38.84,38.75,32.14,31.53,26.83,26.62,22.79,22.24,21.04,18.92,15.37,14.20.hrms(dart-tof)calculatedforc50h61cln9o10s[m+h]+m/z1014.3951,found1014.3975.目标产物g2的合成:该目标产物制备方法同g1,原料为g2-m,其它原料和处理方法相同,产率为28%。1hnmr(400mhz,cdcl3)δ=9.52(s,1h),8.58(d,j=8.3,1h),8.16(s,1h),7.99(s,1h),7.92(dd,j=8.0,1.4,1h),7.77–7.69(m,1h),7.66–7.59(m,1h),7.58(s,1h),7.56–7.52(m,1h),7.49(t,j=5.4,1h),7.25(d,j=8.0,1h),7.19(d,j=8.4,1h),6.75(s,1h),5.07–4.92(m,1h),4.67–4.59(m,2h),4.60–4.47(m,1h),3.51–3.11(m,7h),2.97–2.67(m,6h),2.48(t,j=6.0,2h),2.28(dd,j=22.1,11.6,2h),2.18(d,j=21.2,4h),1.90–1.67(m,4h),1.55(ddd,j=26.9,13.5,6.7,5h),1.34(dd,j=15.3,6.5,12h).13cnmr(101mhz,cdcl3)δ=172.42,171.67,171.13,168.61,166.71,166.67,166.10,157.47,155.34,155.29,154.50,144.80,138.48,137.01,134.64,133.59,131.26,127.84,127.15,124.86,123.61,123.12,120.95,119.65,118.21,117.35,110.97,105.82,71.73,68.10,60.38,55.48,54.20,53.82,49.36,39.11,38.97,37.67,32.40,32.15,31.52,29.54,29.05,26.69,26.39,22.71,22.23,21.04,18.91,15.36,14.20.hrms(dart-tof)calculatedforc52h65cln9o10s[m+h]+m/z1042.4264,found1042.4293.目标产物g3的合成:该目标产物制备方法同g1,原料为g3-m,其它原料和处理方法相同,产率为21%。1hnmr(400mhz,cdcl3)δ=9.52(s,1h),8.58(d,j=8.2,1h),8.16(s,1h),7.99(s,1h),7.93(dd,j=8.0,1.5,1h),7.73(dd,j=10.1,5.7,1h),7.62(dd,j=10.4,3.2,2h),7.55(t,j=6.0,1h),7.39(t,j=5.6,1h),7.26–7.23(m,1h),7.19(d,j=8.4,1h),6.75(s,1h),4.96(dd,j=12.3,5.3,1h),4.63(t,j=7.8,2h),4.59–4.45(m,1h),3.29(dddd,j=27.1,23.9,16.5,8.6,7h),2.98–2.67(m,6h),2.49(d,j=5.7,2h),2.23(d,j=21.1,2h),2.20–2.13(m,3h),1.89–1.69(m,4h),1.62–1.49(m,4h),1.44–1.29(m,18h).13cnmr(101mhz,cdcl3)δ=171.39,168.35,166.68,166.61,166.01,157.46,155.37,155.27,154.49,144.86,138.48,137.03,134.63,133.61,131.28,124.91,123.63,123.13,120.94,119.49,118.13,117.33,110.93,105.87,71.71,68.00,55.48,53.85,49.39,39.09,31.58,29.70,29.35,29.12,29.01,26.78,26.62,22.59,22.21,18.93,15.37.hrms(dart-tof)calculatedforc54h69cln9o10s[m+h]+m/z1070.4577,found1070.4604.9、h系列protac分子的结构与合成中间体h1-m的合成:该中间体的制备方法同g1-m,原料为[2-(2-氨基乙氧基)乙基]氨基甲酸叔丁酯,其他原料和处理方法相同。产率为62%。1hnmr(400mhz,cdcl3)δ=7.78–7.71(m,1h),7.52(t,j=9.6,1h),7.21(t,j=8.5,1h),4.71–4.60(m,2h),3.71–3.47(m,6h),3.35(s,2h),2.98–2.67(m,3h),2.26–2.08(m,1h),1.51–1.31(m,9h).hrms(dart-tof)calculatedforc24h30n4o9na[m+na]+m/z541.1910,found541.1933.中间体h2-m的合成:该中间体的制备方法同g1-m,原料为2-(2-(2-氨基乙氧基)乙氧基)乙基氨基甲酸叔丁酯,其他原料和处理方法相同。产率为51%。1hnmr(400mhz,cdcl3)δ=7.74(dd,j=8.3,7.5,1h),7.54(d,j=7.2,1h),7.21(d,j=8.4,1h),5.06–4.95(m,1h),4.69(d,j=17.6,2h),3.74–3.48(m,10h),3.32(dd,j=28.2,4.0,2h),2.96–2.71(m,3h),2.23–2.09(m,1h),1.40(d,j=21.6,9h).hrms(dart-tof)calculatedforc26h34n4o10na[m+na]+m/z585.2173,found585.2199.中间体h3-m的合成:该中间体的制备方法同g1-m,原料为13-氨基-5,8,11-三氧杂-2-氮杂十三烷酸1,1-二甲基乙酯,其他原料和处理方法相同。产率为41%。1hnmr(400mhz,cdcl3)δ=7.78–7.70(m,1h),7.54(d,j=7.3,1h),7.20(d,j=8.4,1h),4.98(dd,j=12.0,5.4,1h),4.66(s,2h),3.84–3.50(m,15h),3.30(s,2h),2.99–2.69(m,3h),2.22–2.11(m,1h),1.43(s,9h).hrms(dart-tof)calculatedforc28h38n4o11na[m+na]+m/z629.2435,found629.2440目标产物h1的合成:该目标产物的制备方法同g1,原料为h1-m,其他原料和处理方法相同。产率为23%。1hnmr(400mhz,cdcl3)δ=9.51(s,1h),8.58(d,j=8.1,1h),8.15(d,j=4.4,1h),7.99(s,1h),7.92(dd,j=8.0,1.5,1h),7.76–7.71(m,1h),7.67(d,j=4.9,1h),7.64–7.59(m,1h),7.58–7.50(m,3h),7.25(dd,j=9.5,2.3,1h),7.22–7.16(m,1h),5.05–4.92(m,1h),4.63(s,2h),4.54(dt,j=12.1,6.0,1h),3.68–3.42(m,9h),3.33–3.13(m,4h),2.91–2.62(m,7h),2.50(s,2h),2.14(s,3h),1.79(s,4h),1.37–1.29(m,14h).13cnmr(101mhz,cdcl3)δ=171.67,171.12,168.96,166.71,166.67,166.15,157.48,155.32,154.28,144.77,138.49,137.02,134.65,133.66,131.25,127.78,127.08,124.86,123.63,123.11,120.82,119.16,117.97,117.25,111.07,105.78,71.75,69.58,69.37,67.64,60.38,55.47,54.09,53.95,53.84,49.39,39.37,38.83,37.73,32.72,32.05,31.52,29.68,22.91,22.24,21.04,18.92,15.37,14.20.hrms(dart-tof)calculatedforc50h61cln9o11s[m+h]+m/z1030.3900,found1030.3875.目标产物h2的合成:该目标产物的制备方法同g1,原料为h2-m,其他原料和处理方法相同。产率为26%。1hnmr(400mhz,cdcl3)δ=9.51(s,1h),8.58(d,j=8.0,1h),8.21–8.09(m,1h),7.98(s,1h),7.92(dd,j=8.0,1.5,1h),7.73(dd,j=8.3,7.5,1h),7.65–7.59(m,2h),7.58(s,1h),7.53(d,j=7.2,1h),7.27(d,j=9.8,1h),7.19(d,j=8.4,1h),6.79(s,1h),5.04–4.93(m,1h),4.66(d,j=14.3,2h),4.60–4.51(m,1h),3.73–3.52(m,10h),3.45(dd,j=10.7,5.3,2h),3.27(dq,j=13.7,6.8,1h),3.16(d,j=10.6,2h),2.92–2.63(m,6h),2.49(s,2h),2.18–2.12(m,4h),1.79(s,3h),1.38–1.28(m,12h).13cnmr(101mhz,cdcl3)δ=171.63,171.12,168.67,166.89,166.66,165.92,157.49,155.33,155.29,154.43,144.81,138.49,136.98,134.65,133.68,131.25,127.72,127.03,124.84,123.62,123.10,120.86,119.38,118.03,117.29,111.12,105.76,71.67,70.33,69.92,69.53,67.95,60.38,55.47,54.13,53.94,49.35,39.05,39.00,37.84,32.75,32.34,31.52,22.71,22.23,21.04,18.93,15.36,14.20.hrms(dart-tof)calculatedforc52h65cln9o12s[m+h]+m/z1074.4162,found1074.4153.目标产物h3的合成:该目标产物的制备方法同g1,原料为h3-m,其他原料和处理方法相同。产率为25%。1hnmr(400mhz,cdcl3)δ=9.44(s,1h),8.51(d,j=8.3,1h),8.09(d,j=1.3,1h),7.91(s,1h),7.85(dd,j=7.9,1.5,1h),7.72(d,j=4.9,1h),7.65(dd,j=10.7,5.0,1h),7.59(s,1h),7.57–7.53(m,1h),7.52(s,1h),7.45(dd,j=7.3,2.0,1h),7.20–7.15(m,1h),7.15–7.08(m,1h),6.76–6.66(m,1h),4.90(dd,j=11.9,5.4,1h),4.57(s,2h),4.47(dt,j=12.1,6.1,1h),3.78–3.44(m,16h),3.38(s,2h),3.09(d,j=10.3,2h),2.90–2.54(m,7h),2.08(s,4h),1.25(dd,j=10.5,6.5,13h).13cnmr(101mhz,cdcl3)δ=172.37,171.76,168.78,166.92,166.64,165.91,157.46,155.31,155.28,154.43,144.78,138.45,137.03,134.69,133.62,131.25,127.66,127.04,124.78,123.61,123.13,120.86,119.42,117.99,117.27,111.02,105.73,71.60,70.32,70.20,69.93,69.43,67.89,60.40,55.45,54.29,53.94,49.28,39.06,38.95,37.76,32.62,32.32,31.45,29.69,22.73,22.22,21.07,18.96.hrms(dart-tof)calculatedforc54h69cln9o13s[m+h]+m/z1118.4424,found1118.4403.对比化合物l10a的合成:中间体l10的合成:以癸二酸为原料(202mg,1mmol),溶于20mldmf中,加入hatu(1140mg,3mmol),dipea(774mg,6mmol),ldk378(279mg,0.5mmol)常温搅拌10h。tlc监测反应进展,反应完成后,用30ml水淬灭反应,用ea萃取水层后,合并有机层,用饱和食盐水反洗后用无水硫酸钠干燥,减压浓缩后,用dcm/meoh作为流动相进行柱层析分离。得白色固体,产率约为53%。1hnmr(400mhz,cdcl3)δ=9.54(s,1h),8.57(d,j=8.3,1h),8.14(s,1h),8.00–7.88(m,2h),7.75(s,1h),7.66–7.54(m,1h),7.29–7.25(m,1h),6.69(s,1h),4.83(d,j=12.9,1h),3.36–3.09(m,2h),2.34(ddd,j=20.8,15.2,7.6,7h),2.18(s,3h),1.74–1.50(m,10h),1.39–1.28(m,25h).13cnmr(101mhz,cdcl3)δ=178.57,174.34,172.00,157.37,155.45,154.82,145.15,138.39,136.96,134.62,131.27,127.72,126.81,124.93,123.68,123.20,121.31,110.97,105.71,71.65,55.52,51.46,46.64,42.68,38.62,38.35,34.12,34.07,33.44,32.28,29.68,29.42,29.31,29.16,29.07,29.02,25.39,24.89,24.74,22.22,22.14,18.95,15.35.hrms(dart-tof)calculatedforc38h53cln5o6s[m+h]+m/z742.3405,found742.3401.称取l10(137mg,0.2mmol),溶于10mldmf中,加入hatu(91mg,0.24mmol),dipea(73mg,0.6mmol),(2s,4r)-1-((s)-2-amino-3,3-dimethylbutanoyl)-4-hydroxy-n-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide(86mg,0.2mmol)常温搅拌15h。tlc监测反应进展,反应完成后,用30ml水淬灭反应,用ea萃取水层后,合并有机层,用饱和食盐水反洗后用无水硫酸钠干燥,减压浓缩后,用dcm/meoh作为流动相进行柱层析分离。得白色固体,产率约为19%。1hnmr(400mhz,cdcl3)δ=9.51(s,1h),8.68(s,1h),8.58(d,j=8.4,1h),8.15(s,1h),8.02(s,1h),7.93(dd,j=7.9,1.4,1h),7.62(t,j=7.2,1h),7.56(s,1h),7.35(q,j=8.5,5h),6.68(s,1h),6.17(d,j=8.7,1h),4.88–4.69(m,3h),4.65–4.49(m,5h),4.36(d,j=4.6,1h),3.61(dd,j=11.3,3.4,1h),3.30–3.22(m,1h),2.51(s,4h),2.42–2.32(m,3h),2.24–2.13(m,7h),2.04(s,1h),1.70–1.50(m,10h),1.40–1.25(m,34h),0.94(s,11h).13cnmr(101mhz,cdcl3)δ=173.87,172.03,171.70,170.70,157.45,155.36,150.31,148.47,144.78,138.50,138.12,136.64,134.61,131.30,130.98,129.54,128.14,127.90,126.91,124.95,123.64,123.12,120.79,71.68,70.06,58.44,57.48,56.73,55.48,43.28,42.57,38.37,36.41,35.90,34.84,33.46,29.70,29.29,29.17,28.99,26.42,25.46,25.37,22.29,22.21,18.97,16.04,15.37.hrms(dart-tof)calculatedforc60h81cln9o8s2[m+h]+m/z1154.5338,found1154.5331.对比化合物l11a的合成:中间体l11的合成:中间体l11的合成步骤参照l10,但将原料换为十一烷二酸,产率约为48%1hnmr(400mhz,cdcl3)δ=9.55(s,1h),8.56(d,j=8.3,1h),8.15(s,1h),8.00–7.88(m,2h),7.72(d,j=4.0,1h),7.65–7.55(m,1h),7.24(s,1h),6.67(d,j=16.2,1h),4.63–4.48(m,1h),4.00(d,j=13.1,1h),3.34–3.09(m,2h),2.62(dd,j=23.1,10.4,1h),2.47–2.26(m,4h),2.20(d,j=21.4,3h),1.84(dd,j=27.8,15.0,3h),1.71–1.53(m,6h),1.33(dt,j=25.6,12.0,25h).13cnmr(101mhz,cdcl3)δ=178.54,175.99,172.08,157.34,155.47,154.83,145.11,138.39,136.92,134.61,131.29,127.73,126.82,124.99,123.69,123.22,121.22,110.95,105.78,71.68,55.52,46.67,42.70,38.63,38.35,34.01,33.51,32.28,29.70,29.53,29.42,29.24,29.15,29.07,28.97,25.42,24.69,22.24,22.16,20.76,18.95,15.36.hrms(dart-tof)calculatedforc39h55cln5o6s[m+h]+m/z756.3562,found756.3561.该化合物的合成步骤参照l10a,但将原料换为l11,产率约为22%。1hnmr(400mhz,cdcl3)δ=9.51(s,1h),8.67(s,1h),8.58(d,j=8.3,1h),8.16(s,1h),8.03(s,1h),7.93(dd,j=8.0,1.5,1h),7.66–7.58(m,1h),7.55(s,1h),7.39–7.31(m,5h),7.24(s,1h),6.68(d,j=2.8,1h),4.76(t,j=8.1,2h),4.63–4.46(m,4h),4.34(dd,j=14.9,4.6,1h),4.11(d,j=11.2,1h),3.96(d,j=13.4,1h),3.60(dd,j=11.3,3.4,1h),3.26(dt,j=13.6,6.8,1h),2.66–2.49(m,5h),2.36(d,j=6.8,2h),2.17(s,4h),1.80(t,j=14.0,2h),1.74–1.46(m,8h),1.40–1.28(m,13h),0.92(d,j=20.4,10h).13cnmr(101mhz,cdcl3)δ=173.56,172.13,171.13,170.71,157.45,155.35,150.30,148.49,144.74,138.50,138.12,136.51,134.61,131.59,131.30,131.00,129.54,128.15,127.94,126.91,124.95,123.63,123.12,120.75,110.86,105.91,71.69,70.11,58.41,57.77,56.87,55.48,46.43,43.27,42.68,38.33,35.97,34.86,33.38,32.80,32.26,26.44,25.46,24.34,22.29,18.97,16.05,15.37.hrms(dart-tof)calculatedforc60h81cln9o8s2[m+h]+m/z1168.5495,found1168.5491.试验例1对alk的激酶活性测试化合物浓度梯度的配制:受试化合物测试浓度为200nm和20nm,在96孔板中稀释成100倍终浓度的100%dmso溶液,然后用1×kinasebuffer将各浓度的化合物进一步稀释成5倍终浓度的中间稀释溶液。化合物测试浓度为200nm起始,5倍稀释10个浓度,单孔测试,同样在96孔板中稀释成100倍终浓度10个浓度梯度的100%dmso溶液,然后用1×kinasebuffer将各浓度的化合物进一步稀释成5倍终浓度的中间稀释溶液。将配制好的化合物溶液各取5μl分别加入384孔板的化合物孔,每个浓度单孔测试;阴性对照孔和阳性对照孔中分别加5μl的5%dmso。用1×kinasebuffer配制2.5倍终浓度的激酶溶液。在化合物孔和阳性对照孔分别加10μl的2.5倍终浓度的激酶溶液;在阴性对照孔中加10μl的1×kinasebuffer。1000rpm离心30秒,振荡混匀后室温孵育10分钟。用1×kinasebuffer配制2.5倍终浓度的atp和kinasesubstrate22的混合溶液。加入10μl的2.5倍终浓度的atp和底物的混合溶液,起始反应。将384孔板1000rpm离心30秒,振荡混匀后28度孵育25分钟。加入25μl终止检测液停止激酶反应,1000rpm离心30秒,振荡混匀用caliperezreaderⅱ读取转化率。数据分析:计算公式%inhibition=(conversion%_max-conversion%_sample)/(conversion%_max-conversion%_min)×100%其中:conversion%_sample是样品的转化率读数;conversion%_min:阴性对照孔均值,代表没有酶活孔的转化率读数;conversion%_max:阳性对照孔比值均值,代表没有化合物抑制孔的转化率读数。计算结果见表1。表1化合物分子在200nm和20nm下对alk的抑制率(%)编号抑制率(200nm)抑制率(20nm)编号抑制率(200nm)抑制率(20nm)l4p95.319.1c295.22.3l6p99.847.2c396.681.4l7p9420.4d199.593.9l12p99.398.2d297.694.8a196.856.9d3101.494.9a297.973.2e1100.699.7a394.553.8e2101.180.4a493.835.6e399.872.6a597.392.7f1100.9100.7a693.691.5f298.399.2a798.657.9g1100.764.9b1101.187.7g298.998.4b299.697.2g3100.391.8b3100.898.4h1100.597.6b4100.679.1h299.7100.3b599.691.3h399.3100.5b699.185.1ldk37899.899.6b799.381.2l10a64.427.2c199.289.1l11a71.47.2从表1可以看出,本发明化合物对alk的抑制率与阳性药ldk378相当,可见,接入本发明的特定的e3连接酶配体不会在很大程度上影响药物分子对alk的激酶活性,而接入其他配体,比如接入vhl抑制剂类的protac分子,将会造成对alk的抑制活性急剧下降。测定b3对alk抑制率与浓度的关系,其结果见表2,根据表2数据,以浓度的log值作为x轴,百分比抑制率为y轴,采用分析软件graphpadprism5的log(inhibitor)vs.response-variableslope拟合量效曲线,从而得出b3化合物对酶活性的ic50值为0.7nm。表2b3浓度(nm)logcon.%inhibition2002.30102999699.2401.602059991101.380.90308998798.21.60.20411998372.10.32-0.49485002228.50.064-1.1938200266.00.0128-1.89279003-1.30.00256-2.5917600359.80.000512-3.290730039-4.40.0001024-3.989700043-7.4试验例2对肿瘤细胞的抑制活性采用mtt法测定本发明的化合物在体外对肿瘤细胞的抑制活性,本实验所用的细胞株包括:h3122(人类非小细胞肺癌细胞株),h2228(人类非小细胞肺癌细胞株),h1299(人肺癌细胞),a549(人肺癌细胞),hela(人宫颈癌细胞)。实验方法为:取对数生长期肿瘤细胞,用胰酶消化,离心并用新鲜培养基重悬,将细胞按照合适的密度接种于96孔板中,每孔培养基100μl。将接种好后,将96孔板置于孵箱中继续培养24h,使肿瘤细胞贴壁。培养环境:温度37℃,5%co2。待细胞贴壁并达到合适细胞密度时,加药,初筛的浓度设置为20、10、5μm,精筛的浓度设计为10、5、2.5、1.25、0.625、0.3μm。72h后停止实验,加入20μlmtt溶液(5mg/ml),放入细胞培养箱中作用4h。取出孔板,弃上清液,每孔再加入150μldmso以溶解甲瓒。待结晶充分溶解后,使用酶标仪测定每孔在490和570nm的吸光度值(od值),计算各个实验组中肿瘤细胞体外增殖抑制率和细胞存活率。根据公式:相对细胞增殖抑制率(%)=(空白对照组-实验组)/空白对照组×100%。每组设置3个平行复孔,每个实验重复三次。实验结果见表3。表3:化合物的体外抗肿瘤活性(ic50:μm)编号h3122h2228h1299a549helal12p>20>20>207.547.98b1>20>20>2054.57b20.73.57.592.62.23b30.30.92.841.61.18b48.37>20>20>20>20c13.528.7>2013.388.15c21.13.1>205.134.36c32.6615.1>20>207.67d11.835.747.236.735.78d22.344.5110.865.815.86d3>20>20>20>2012.21e1>207.6>2014.349.05f14.865.212.2820.97.98f2>205.714.96.015.52g11.3>209.33>206.99g2>20>2018.834.663.87h15.4112.910.16>207.94ldk3781.11.32.921.320.94l10a>20>20>20>20>20l11a>20>20>20>20>20从表3可以看出,本发明的化合物采用不同的linker进行偶联,有多个分子具有体外抗肿瘤活性。其中,化合物b2和b3对h3122的抑制活性超过了阳性药ldk378。而大部分的化合物的体外抗肿瘤活性均超过了对比化合物l10a和l11a,可见,本发明采用泊马度胺类的e3连接酶配体,其体外抗肿瘤活性大于采用vhl抑制剂类的protac分子,vhl抑制剂类的protac分子未能在细胞层面上抑制alk活性或有效降解alk而起到抑制细胞增殖的作用。试验例3对正常细胞的毒性试验研究采用mtt法,测定了部分抗肿瘤活性较好的化合物分子对lo2(人正常肝细胞系)在5μm给药浓度下的细胞毒性。其结果见表4。表4编号抑制率编号抑制率b233.57%d132.36%b363.22%d252.78%c129.77%f265.48%c254.47%g259.43%c343.55%ldk37868.47%实验结果表明,在同等浓度下,protac分子对lo2的抑制率均低于阳性药ldk378。说明了protac分子具有一定的选择性,安全性也较好。试验例4b3分子蛋白质印迹采用wb的方法,对protac分子处理过的细胞中alk及相关蛋白的量进行考察。实验方法:细胞处理和收集:本实验在6孔板中进行。取对数生长期细胞,用胰酶消化,离心并用新鲜培养基重悬,将细胞按照合适的密度接种于6孔板中,每孔培养基体积1ml。细胞贴壁后按一定的浓度梯度加入b3分子或阳性药ldk378,处理12h后收集细胞,用预冷的pbs洗2次,然后在转速1200rpm下离心3min,弃上清液;再次离心,弃上清液。细胞裂解:将6孔板置于冰上,加入适当体积的ripa裂解液,作用30min。ripa裂解液中含有1%的cocktail蛋白酶抑制剂以及1%的磷酸化酶抑制剂。之后用细胞超声破碎仪将细胞裂解液置于冰上超声3次,每次3秒,间隙2秒。超声功率:35%,温度控制:20℃。将细胞裂解液置于4℃离心机中离心15min,转速13300rpm。收集上清,用nanodrop2000仪器测定每组样品的蛋白浓度。然后使用ripa裂解液将蛋白浓度调整至适宜的浓度,加入蛋白上样缓冲液,沸水煮样品5-10min。离心1min,转速133000rpm,将样品置于-20℃冰箱中冷冻保存。sds-page凝胶电泳操作:根据实验需要,我们使用8-15%的聚丙烯酰胺凝胶分离蛋白。上样:将配置好的胶板安装于电泳槽中,加入电泳缓冲液。使用移液枪将蛋白marker和样品分别加入到上样孔中。电泳:连接电源,将电压调至80v,恒压跑sds-page凝胶电泳,直至样品跑到浓缩胶和分离胶的分界线处,且蛋白marker条带略微分开。将电压提高至120v,恒压跑sds-page凝胶电泳。直至蛋白预染marker到达所需位置,终止电泳。取出凝胶并开始转膜。转膜:取出加在玻璃板内的凝胶,按照实验所需蛋白位置截取合适的凝胶,将其放置于转膜缓冲液中。剪出合适大小的pvdf膜,按照顺序装配转膜“三明治”装置,即海绵—3-4层滤纸—凝胶—pvdf膜—3-4层滤纸—海绵,将每层按顺序放好后,夹紧装置,安装于转移槽中,加满转膜缓冲液。将装置置于冰浴中,接通电源开始转膜。恒压100v;转膜时间45min。转膜结束后,取出pvdf膜,放于tbs缓冲液中。免疫杂交和显色:1)1×tbs洗膜2次,每次5min,室温摇床缓慢摇动。2)将pvdf膜置于封闭缓冲液中,室温摇床缓慢摇动,封闭2h。3)用1×tbs/t缓冲液洗膜3次,每次5min。4)将pvdf膜置于杂交袋中,加入合适浓度的一抗。4℃摇床缓慢摇动,孵育一抗过夜。5)次日,取出孵育过一抗的pvdf膜,1×tbs/t洗膜3次,每次5min。6)将膜置于含相应二抗的杂交袋中,37℃置于摇床孵育1h,使用tbs/t缓冲液洗膜3次,每次15min,最后再用tbs缓冲液洗膜一次,时间为5min。7)显影:按照发光法进行显影。发光反应时将试剂盒中两种溶液按1:1比例混合,将pvdf膜浸没于溶液中,静置一段时间,上机检测。最后对条带进行分析,使用gapdh作内参。实验结果见图1。根据wb结果发现,protac分子能够有效降解细胞内部的alk,且化合物分子对于alk的降解能力呈现浓度相关性。证明了这些分子可以在细胞内实现诱导降解alk蛋白。通过影响alk蛋白的含量,细胞内p-s6的含量也出现了降低。试验例5pi/annexinv双染检测凋亡磷脂酰丝氨酸是一种带负电荷的磷脂结构,正常情况下主要存在于细胞膜的内侧面,在细胞发生凋亡的时候,磷脂酰丝氨酸会转移到细胞膜外。annexinv是一种钙离子依赖性磷脂结合蛋白,能与膜外磷脂酰丝氨酸高亲和力特异性结合。但ps转移到细胞膜外这一现象也可发生在细胞坏死中。这两种细胞死亡方式间的差别是在凋亡的初始阶段细胞膜是否完好,坏死细胞在其早期阶段细胞膜的完整性就被破坏了。propidiumiodide(pi)是一种核酸染料,它不能透过完整的细胞膜,但在凋亡中晚期的细胞和死细胞,pi能够透过细胞膜与细胞核结合呈现红色。所以将annexinv与pi匹配使用,可以将凋亡早期的细胞和晚期的细胞以及死细胞区分开来。实验器材:预冷pbs缓冲液,0.25%不含edta胰酶,离心机,annexinv/pi双染试剂盒,流式细胞仪。细胞收集:取对数生长期的h3122细胞,用0.25%不含edta的胰酶消化并用吸管吹打成单个细胞悬浮细胞直接收集到10ml的离心管中,每样本细胞数为(1-5)×106个/ml,1500r/min离心5min,弃去培养液。之后,用预冷的pbs缓冲液洗涤2次,1500r/min离心5min,弃上清液。染色:根据annexinv/pi双染试剂盒说明书,加入195μl的结合液重悬细胞,混匀;加入5μl的annexinv-fitc混匀后,避光,室温孵育15分钟,再加入5μl的pi染色5-15分钟后,上机检测。流式细胞仪分析:流式细胞仪激发光采用488nm波长段,用一波长为515nm的通带滤器来检测fitc荧光,另一波长大于560nm的滤器检测来pi。试验结果见图2。从图2可以看出,在双变量的散点图上,左下象限为双阴结果(fitc-/pi-),代表活细胞;右上象限为双阳结果为(fitc+/pi+)表示坏死细胞或晚期凋亡细胞;而右下象限为fitc阳性,pi阴性,代表早期凋亡的细胞。从图中我们可以明显看出:h3122细胞的凋亡率随着目标化合物b3浓度的增加而逐步增加,在20μm浓度时,早期凋亡率达到34.9%,故可推测目标化合物b3能诱发非小细胞肺癌株h3122发生凋亡,并具有浓度依数性。试验例6分子荧光特性测试采用了荧光酶标仪测定本发明分子的吸收波长和激发波长。具体测定方法如下:使用荧光酶标仪进行波长扫描,将b3溶解于二甲亚砜中,量取100微升置于96孔板,设定的吸收波长扫描范围为300-700nm,得到一个b3分子的吸收谱图,详见图3。从化合物分子的吸收谱图情况,可以看出该化合物在390-450nm波长均有较强的紫外吸收,最大的吸收波长为420nm。因此,以420nm波长作为激发波长,发射波长的扫描范围为450nm-700nm波长。结果如图4。从图4可以发现,b3分子在520nm波长下有最强的荧光强度。综上所述,化合物b3的激发波长为420nm,发射波长为520nm。根据该化合物分子的荧光特性,观察化合物分子在细胞内或动物体内的分布,在6孔板中接种合适密度的h2228细胞,待细胞贴壁后,按顺序加入20μm,10μm,5μm,2.5μm,1.25μm的b3及等量dmso。培养48h后,将6孔板置于荧光倒置显微镜下观察,六孔板中a-f的六个孔对应的b3的浓度为20μm,10μm,5μm,2.5μm,1.25μm的b3及等量dmso。在紫光下观察(4倍镜头),蓝色荧光的强度明显随浓度的下降而减弱,而视野中细胞的密度随药物浓度的降低而增大。化合物b3分子在细胞内的分布见图5。在放大倍数为20倍时,用紫光可以明显看到化合物在细胞内富集,且浓度远大于胞外的培养基,图5为放大倍数40倍时,紫光与白光下效果的叠合图,可以明显观察到,荧光区域主要集中在细胞内的胞浆区域,说明b3主要分布在细胞质中。通过研究发现,b3分子的荧光区域也是一个平面区域,可能由于分子间π-π作用使分子荧光减弱,在细胞内或细胞外与alk蛋白结合后,分子间的π-π作用减弱,可以使与蛋白结合的分子荧光强于游离状态,从而标记alk蛋白的位置。alk蛋白作为一个致癌蛋白,不同细胞内该蛋白含量不同,根据加药后细胞内荧光强度的不同,可以判断胞内alk蛋白含量。为了判断分子细胞活性与alk蛋白含量的关系,在三个培养皿中培养了等量的细胞,分别为lo2,h1299,h3122,分别加入1.25μm的b3,用荧光倒置显微镜观察细胞内荧光的强弱,其结果如图6所示。可见,蛋白的含量与分子对细胞的活性是一致的,说明该分子可以用于分子示踪,也可以标记细胞内alk蛋白的含量。试验例7b3分子体内抗肿瘤活性研究为了研究protac分子在动物体内的抗肿瘤活性,我们对化合物分子b3进行了体内抗肿瘤活性研究,选用的细胞株为h3122,实验动物为balb/cnude小鼠。实验动物:所用小鼠购于北京华阜康生物科技股份有限公司,饲养于四川大学生物治疗国家重点实验室动物房(spf级)。小鼠为spf级,按照nationalinstitutesofhealthguideforthecareanduseoflaboratoryanimals标准饲养。实验动物的使用由四川大学伦理委员会(institutionalethicscommitteeofsichuanuniversity)审议批准。实验动物饲养条件:温度16-26℃,相对湿度40%-70%,采用人工照明系统,12h明暗交替,co60灭菌饲料(北京科澳协力饲料有限公司),自由饮水。移植肿瘤动物模型建立:移植性肿瘤动物模型分为同种移植和异种移植,是将动物或人源肿瘤移植到同种或异种动物体内增殖而形成肿瘤。异种动物移植常将肿瘤细胞接种于裸鼠皮下,该方法成瘤率高,成瘤时间均一,瘤体大小和肿瘤重量易于检测。在本课题中,我们建立移植性h3122肿瘤小鼠动物模型,旨在评价化合物b3的体内抗肿瘤效果。具体方法如下:1)胰酶消化对数生长期的h3122肿瘤细胞后,离心收集细胞;用双无(无抗生素无血清)培养基清洗细胞3次;加入适量双无培养基重悬肿瘤细胞,并对肿瘤细胞进行密度测定;根据实验要求将细胞密度调至5×106个/ml。2)将重悬好的细胞按照每只小鼠0.5×106个细胞(0.1ml)将细胞接种到小鼠(6-8周龄,体重18-22g)的右腋部皮下。3)接种后10天左右,小鼠接种部位肿瘤成瘤,体积150-200mm3时,将所有肿瘤体积相近的小鼠随机分组。实验设置2组:控制组和给药组(b310mg/kg,20mg/kg,40mg/kg,100mg/kg;阳性对照ldk37820mg/kg),每组7只。药物溶媒采用peg300:nmp=9的混合溶媒,每只裸鼠每天平均口服给药0.2ml。实验数据记录:每3天测量一次肿瘤体积和小鼠体重,包括肿瘤长径和垂直于长径的短径,单位:毫米(mm);小鼠体重,单位:克(g)。实验期间需要注意观察小鼠健康状况,如动物活动情况、进水进食情况、小鼠毛发光泽度及颜色、有无腹泻和肿瘤部位有无炎症等。瘤体积(mm3)=肿瘤长径×短径×短径/2抑瘤率(%)=(对照组肿瘤平均体积-实验组肿瘤平均体积)/对照组肿瘤平均体积×100%。实验进行15天。实验结束后,使用水合氯醛麻醉处死小鼠,取出小鼠心、肝、脾、肺和肾等脏器,同时,剥离肿瘤组织。将主要脏器和肿瘤组织按分组用4%多聚甲醛固定。肿瘤体积随给药时间的变体见图7,裸鼠体重随给药日期的变化见图8。试验结果表明,实验期间小鼠健康状况良好:活动情况正常、进水进食情况正常、小鼠皮肤光泽度及颜色正常、无腹泻现象发生,肿瘤部位无炎症出现。从给药结果来看,化合物b3与控制组组相比能够抑制肿瘤组织增长,化合物b3与控制组相比能够抑制肿瘤组织增长,在给药剂量为10mg/kg,20mg/kg,40mg/kg,100mg/kg时抑瘤率为45.5%,59.1%,61.2%,70.1%;与之相比,阳性药ldk378在给药剂量为20mg/kg时的抑瘤率为49.5%,同等给药剂量下,b3的体内抗肿瘤活性优于阳性药物ldk378。小鼠体重在给药期间比较平稳,各给药组之间差异并不明显。当前第1页1 2 3
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1
- 肿瘤靶向核磁共振/荧光双模态成像造影剂及其制备和应用的制造方法与工艺
- pH氧化还原双敏感的PAMAM靶向纳米给药载体及其制备方法与制造工艺
- 具有卵巢肿瘤靶向D构型多肽及其基因递释系统的制造方法与工艺
- 靶向survivin纳米抗体及其制备方法和应用与制造工艺
- 用于PSMA靶向的成像和放射疗法的金属/放射性金属标记的PSMA抑制物的制造方法与工艺
- 一种线粒体靶向的粘度荧光探针及其制备方法和应用与制造工艺
- 一种兼具荧光可视、磁靶向和pH敏多功能的复合空心微球药物载体及其制备方法与制造工艺
- 一种用于修饰微泡的多肽以及靶向GBM的药物制剂的制造方法与工艺
- 白果内酯作为脑靶向增效剂在制备防治脑肿瘤药物的应用的制造方法与工艺
- 机器人辅助精确靶向穿刺软组织目标柔性针夹紧固定装置的制造方法