一种3,4-二苯基丁酰胺类化合物的合成方法与流程
本发明属于有机合成领域,特别涉及一种3,4-二苯基丁酰胺类化合物的合成方法。
背景技术:
3,4-二苯基丁酰胺及其衍生物作为一类具有多种生物活性的有机化合物,在医药学方面广泛应用,例如医药中间体,抗菌剂,酶抑制剂等。其中3-(2-(二甲氧基甲基)苯基)-4-苯基丁酰胺可以作为一种医药中间体,如sigma受体配体a。n-(3-氯苯基)-3-(4-苯氧基苯基)-4-苯丁胺具有很好的杀菌活性,对于抵抗稻瘟病菌效果显著(b)。4-(4-氟苯基)-n,3-二羟基-3-(4-羟基苯基)-n-甲基丁酰胺类化合物对尿素酶有较好的抑制作用,同时也可以做为药物中间体,用于制备抗胃炎、胃溃疡、尿路结石等药物c。传统的合成二苯基取代丁酰胺化合物的方法需要多步反应导致收率低,合成需要催化剂成本较高,需要过渡金属催化,或者所用辅助原料复杂等。由于以上原因导致二苯基取代的丁酰胺类化合物的合成收率不高,不易实现规模化制备,且污染环境。
本发明中目标产物类似化合物合成已有报道(jderosa,tkang,vttran,etal.angew.chem.int.ed.2020,132(3):1201-1205),具体路线如下:
该方法以丁烯酸、碘苯、苯并[d][1,3]二恶唑作为原料,经过多步反应得到3,4-二苯基取代的丁胺类化合物。此反应需要五步,且最终产物只有33%。
技术实现要素:
本发明的目的在于提供一种解决以上问题,合成方法简单、经济、绿色环保、适用性更加广泛或适于规模化生产的3,4-二苯基丁酰胺类化合物的合成方法。
实现上述目的的技术方案如下:
一种3,4-二苯基丁酰胺类化合物的合成方法,式1所示的不饱和酰胺类化合物和式2所示的甲苯类化合物、强碱和铯盐添加剂,有机溶剂混合,反应合成式3所示的3,4-二苯基丁酰胺类化合物。
其中r1选自苯基、取代苯基、噻吩、1,3-苯并[d][1,3]二恶唑或1-萘基,其中取代苯基的取代基包括甲基、卤素或甲氧基,r2选自甲基或乙基,r3选自氢、卤素、苯基或甲基。本发明方法为一锅法生成3,4-二苯基丁酰胺类化合物,减少了反应步骤,从而可提高产物收率;本合成方法所用原料简单经济,不用使用过渡金属催化剂,更加绿色环保。
优选的,r1选自苯基、甲氧基苯基、甲基苯基,r2选自甲基,r3选自氢。
更加优选的,r1选自卤素苯基,苯并[d][1,3]二氧杂-5-基、噻吩-2-基或萘-1-基,r2选自甲基,r3选自卤素。
优选的,r1为苯基,r2为甲基,r3为甲基。
更加优选的,r1为苯基,r2为甲基,r3为卤素或苯基。
优选的,反应在惰性气体保护下进行。
优选的,反应中式2所示的甲苯类化合物、式1所示的不饱和酰胺类化合物、强碱和铯盐添加剂的摩尔比为:566∶10∶2~10∶1~5,反应温度为105℃~115℃。
优选的,反应温度为110℃。
优选的,强碱为二(三甲基硅基)氨基锂。
优选的,有机溶剂为n,n-二甲基甲酰胺。
优选的,铯盐添加剂为氟化铯、特戊酸铯。
优选的,铯盐添加剂为氟化铯。
优选的,惰性气体为氩气或氮气。
优选的,采用本发明方法,可以合成如下结构的3,4-二苯基丁酰胺类化合物:
在强碱和铯盐添加剂的存在下,甲苯去质子化,得到苄基碳负离子和共轭酸,然后苄基碳负离子对肉桂酰胺进行1,4-加成得到产物碱中间体,中间体夺取共轭酸中的质子产生目标产物。
采用本发明的技术方案至少可以达到如下有益效果之一:
本发明的合成方法未用到过渡金属催化剂,绿色环保;
本发明采用一锅法合成方法,由于反应步骤少,减少了原料的损失,提高了产物的产率;
本发明所需操作步骤较为简便,无需极端的升温或降温,更易于操作和控制;
本发明中的r1、r2、r3可以为多种选择,因此本发明方法适用性更加广泛,可以合成多种3,4-二苯基取代的丁酰胺类化合物。
附图说明
图1~25分别为实施例1~25产物的核磁共振谱图
其中每一实施例产物的谱图中,a图为对应实施例产物的氢谱图,b图为对应实施例产物的碳谱图。
具体实施方式
为了便于本领域技术人员理解,下面结合实施例对本发明的构思做进一步的说明。同时,说明书中所涉及的各种原料,均购自市场,二(三甲基硅基)氨基锂(aldrich,97%),氟化铯(aldrich,99%),其他药品等购自sigma-aldrich,acros,alfaaesar,tcichina,adamas-beta或者j&k.,核磁共振谱仪型号为布鲁克400兆。
实施例1
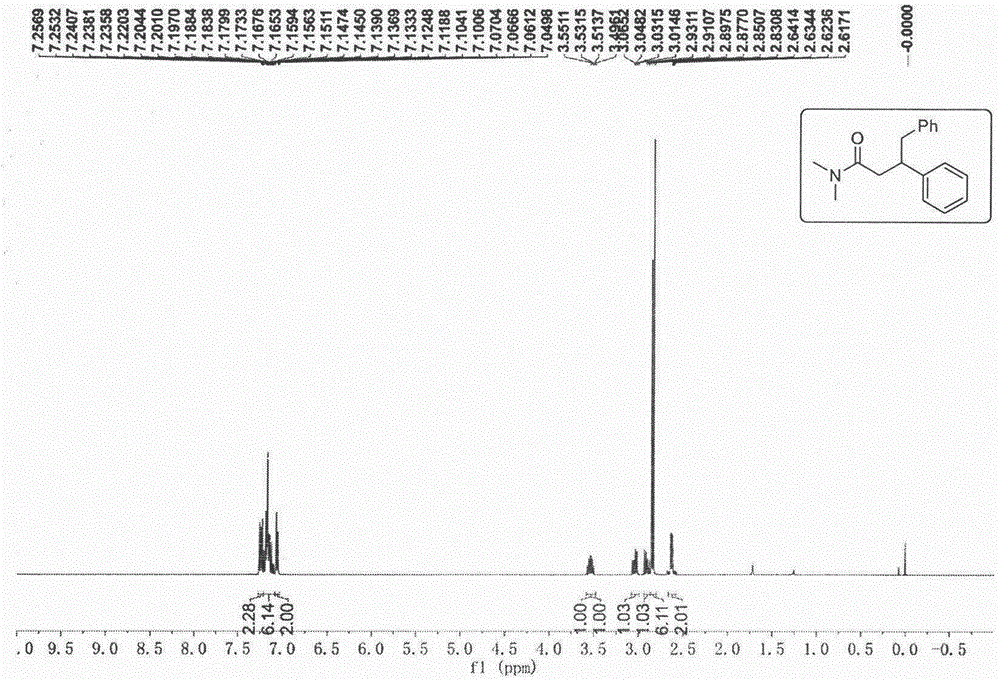
在充满氩气的手套箱中,向一个带有磁子的干燥微波管依次加入n,n-二甲基肉桂酰胺(17.5mg,0.1mmol)、lin(sime3)2(3.3mg,0.02mmol)、csf(1.5mg,0.01mmol)、甲苯(0.6ml)以及dmf(1.2ml),将微波管盖紧处于密封状态拿出手套箱。反应混合物在110℃的搅拌油浴锅中反应12h,然后将密封瓶冷却至室温,打开盖加入三滴水淬灭反应。将反应混合物经过一个短的硅胶柱进行过滤,同时用10ml的乙酸乙酯分批次冲洗,然后将带有乙酸乙酯的混合溶剂进行减压旋蒸得到粗产品,最后用柱层析对粗产品进行分离得到纯净的n,n-二甲基-3,4-二苯基丁酰胺化合物(21.3mg,80%yield)。熔点mp:101.3-102.1℃,产物的氢谱和碳谱核磁共振谱图分别为图1a和图1b,谱图数据为:1hnmr(400mhz,cdcl3)δ:7.27-7.21(m,2h),7.22-7.08(m,6h),7.08-7.03(m,2h),3.53(m,1h),3.04(dd,j=13.5,6.8hz,1h),2.90(dd,j=13.4,8.2hz,1h),2.84(d,j=8.0hz,6h),2.63(dd,j=7.0,2.7hz,2h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.4,144.2,139.9,129.2,128.22,128.0,127.6,126.3,125.9,43.8,42.5,38.9,37.2,35.4ppm
表中列出了1-25各实施例中r1、r2、r3以及对应的产物的结构式,最后一列列出了各实施例的产物的产率,并标示出了各实施例的具体实施条件,各实施例实施条件的具体含义如表格下方所示。
[a]不饱和酰胺类化合物(0.1mmol),甲苯类化合物(0.6ml),二(三甲基硅基)氨基锂(0.02mmol),氟化铯(0.01mmol),n,n-二甲基甲酰胺(1.2ml)
[b]不饱和酰胺类化合物(0.1mmol),甲苯类化合物(0.6ml),二(三甲基硅基)氨基锂(0.02mmol),氟化铯(0.05mmol),n,n-二甲基甲酰胺(1.2ml)
[c]不饱和酰胺类化合物(0.1mmol),甲苯类化合物(0.6ml),二(三甲基硅基)氨基锂(0.1mmol),氟化铯(0.05mmol),n,n-二甲基甲酰胺(1.2ml)
以下为2-25各个实施例产物的1h和13c的核磁共振谱图(nmr)图谱数据结果以及部分产物的熔点mp、高分辨质谱图(hrms)数据结果。
实施例2
实施例2所得产物的收率是(21.9mg,70%yield),熔点mp:118.6-120.8℃。产物的氢谱和碳谱核磁共振谱图分别为图2a和2b,谱图数据为:1hnmr(400mhz,cdcl3)δ:7.22-7.15(m,2h),7.15-7.04(m,5h),6.81-6.75(m,2h),3.76(s,3h),3.48(p,j=7.1hz,1h),3.01(dd,j=13.4,6.7hz,1h),2.88(m,1h),2.84(d,j=7.4hz,6h),2.60(d,j=7.2hz,2h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.6,157.9,140.1,136.2,129.2,128.5,128.0,125.8,113.6,55.1,43.0,42.7,39.2,37.2,35.4ppm.hrms:calcdforc19h24no2[m+h]+298.1807,found298.1803.
实施例3
实施例3所得产物的收率是(22.3mg,78%yield),熔点mp:113.0-114.7℃。产物的氢谱和碳谱核磁共振谱图分别为图3a和3b,谱图数据为:1hnmr(400mhz,cdcl3)δ:7.21-7.16(m,2h),7.15-7.09(m,3h),7.05-7.03(m,2h),6.94-6.89(m,2h),3.57-3.49(m,1h),3.02(dd,j=13.3,6.7hz,1h),2.89-2.82(m,7h),2.61(d,j=7.0hz,2h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.2,161.3(q,j1c-f=244.5hz),139.8(q,j4c-f=3.3hz),139.6,129.1,129.0(q,j3c-f=7.9hz),128.1,126.0,114.9(q,j12c-f=21.1hz),43.1,42.4,39.0,37.1,35.4ppm.hrms:calcdforc18h21fno[m+h]+286.1607,found286.1606.
实施例4
实施例4所得产物的收率是(22.7mg,76%yield),产物的氢谱和碳谱核磁共振谱图分别为图4a和4b,谱图数据为:1hnmr(400mhz,cdcl3)δ:7.22-7.02(m,7h),6.89-6.76(m,2h),3.87(p,j=7.2hz,1h),3.76(s,3h),3.01(dd,j=7.4,5.5hz,2h),2.85(d,j=9.6hz,5h),2.72(dd,j=15.2,6.8hz,1h),2.63(dd,j=15.2,7.3hz,1h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.9,157.2,140.6,132.0,129.2,128.4,127.8,127.2,125.6,120.4,110.6,55.3,40.5,38.1,37.3,37.2,35.3ppm.hrms:calcdforc19h24no2[m+h]+298.1807,found298.1806.
实施例5
实施例5所得产物的收率是(21.8mg,73%yield),熔点mp:62.8-63.7℃,产物的氢谱和碳谱核磁共振谱图分别为图5a和5b,谱图数据为:1hnmr(400mhz,cdcl3)δ:7.21-7.06(m,6h),6.77-6.68(m,3h),3.74(s,3h),3.55-3.48(m,1h),3.02(dd,j=13.5,6.7hz,1h),2.91-2.88(m,1h),2.85(d,j=6.0hz,6h),2.62(dd,j=6.9,5.2hz,2h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.4,159.4,145.9,139.9,129.2,129.1,128.0,125.9,120.0,113.5,111.4,55.1,43.8,42.4,38.8,37.2,35.4ppm.hrms:calcdforc19h24no2[m+h]+298.1807,found298.1811.
实施例6
实施例6所得产物的收率是(18.8mg,67%yield),产物的氢谱和碳谱核磁共振谱图分别为图6a和6b,熔点mp:61.6-64.4℃。谱图数据为:1hnmr(400mhz,cdcl3)δ:7.21-7.17(m,2h),7.15-7.06(m,4h),6.98-6.95(m,3h),3.49-3.46(m,1h),3.01(dd,j=13.5,7.0hz,1h),2.90(dd,j=13.5,7.9hz,1h),2.84(d,j=9.1hz,6h),2.60(dd,j=7.0,3.8hz,2h),2.29(s,3h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.5,144.2,140.1,137.7,129.3,128.5,128.1,128.0,127.0,125.9,124.5,43.7,42.5,38.9,37.2,35.4,21.4ppm.hrms:calcdforc19h24no[m+h]+282.1858,found282.1855.
实施例7
实施例7所得产物的收率是(28.6mg,83%yield),产物的氢谱和碳谱核磁共振谱图分别为图7a和7b,熔点mp:61.4-62.6℃。谱图数据为:1hnmr(400mhz,cdcl3)δ:7.15-7.09(m,4h),7.05-7.03(m,2h),7.00-6.96(m,2h),3.57-3.42(m,1h),3.02(dd,j=13.5,6.3hz,1h),2.88-2.80(m,8h),2.60(dd,j=7.0,1.1hz,2h),1.21(d,j=7.0hz,6h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.4,146.9,141.1,138.7,131.5,130.6,128.1,127.4,126.3,43.3,41.7,39.0,37.1,35.4,33.6,23.96,23.95ppm.hrms:calcdforc21h27clno[m+h]+344.1781,found344.1785.
实施例8
实施例8所得产物的收率是(25.2mg,66%yield),产物的氢谱和碳谱核磁共振谱图分别为图8a和8b,熔点mp:112.7-114.6℃。谱图数据为:1hnmr(400mhz,cdcl3)δ:7.35(d,j=8.3hz,2h),7.15(d,j=8.3hz,2h),6.97(dd,j=18.3,8.3hz,4h),3.51-3.44(m,1h),3.02(dd,j=13.5,6.0hz,1h),2.89(d,j=2.5hz,6h),2.77(dd,j=13.3,8.8hz,1h),2.60(d,j=7.0hz,2h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:170.9,142.8,138.0,131.8,131.4,130.5,129.4,128.2,120.1,43.2,41.7,38.8,37.1,35.4ppm.hrms:calcdforc18h20brclno[m+h]+380.0417,found380.0418.
实施例9
实施例9所得产物的收率是(25.4mg,80%yield),产物的氢谱和碳谱核磁共振谱图分别为图9a和9b,谱图数据为:1hnmr(400mhz,cdcl3)δ:7.22-7.13(m,2h),7.18-7.06(m,2h),7.11-6.99(m,2h),6.98-6.88(m,2h),3.85-3.71(m,1h),3.02(dd,j=13.2,5.7hz,1h),2.89(d,j=3.2hz,6h),2.78(dd,j=13.2,9.0hz,1h),2.63(d,j=6.9hz,2h),2.10(s,3h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.4,142.1,138.5,136.4,131.6,130.5,130.3,128.0,126.0,125.9,125.8,41.8,38.7,38.4,37.1,35.4,19.6ppm.hrms:calcdforc19h23clno[m+h]+316.1468,found316.1467.
实施例10
实施例10所得产物的收率是(30.7mg,89%yield),产物的氢谱和碳谱核磁共振谱图分别为图10a和10b,熔点mp:71.8-73.1℃。谱图数据为:1hnmr(400mhz,cdcl3)δ:7.15(d,j=8.3hz,2h),6.97(d,j=8.2hz,2h),6.67-6.54(m,2h),6.54(dd,j=7.9,1.5hz,1h),5.90(s,2h),3.50-3.26(m,1h),3.00(dd,j=134,5.8hz,1h),2.89(s,6h),2.76(dd,j=13.4,9.0hz,1h),2.58(d,j=4.1hz,2h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.2,147.5,145.9,138.4,137.6,131.6,130.5,128.1,120.8,108.1,107.7,100.8,43.5,41.9,39.3,37.2,35.4ppm.hrms:calcdforc19h21clno3[m+h]+346.1210,found346.1214.
实施例11
实施例11所得产物的收率是(24.3mg,79%yield),产物的氢谱和碳谱核磁共振谱图分别为图11a和11b,谱图数据为1hnmr(400mhz,cdcl3)δ:7.17(d,j=8.3hz,2h),7.15-7.06(m,1h),7.02(d,j=8.3hz,2h),6.89-6.80(m,1h),6.72-6.63(m,1h),3.89-3.81(m,1h),3.08(dd,j=13.5,5.8hz,1h),2.91(d,j=4.2hz,6h),2.84(dd,j=13.5,8.7hz,1h),2.64(dd,j=12.4,6.9hz,2h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:170.8,147.5,138.1,131.8,130.5,128.2,126.5,124.3,123.0,42.8,40.0,39.2,37.1,35.6ppm.hrms:calcdforc16h19clnos[m+h]+308.0876,found308.0875.
实施例12
实施例12所得产物的收率是(18.2mg,54%yield),产物的氢谱和碳谱核磁共振谱图分别为图12a和12b,熔点mp:86.2-87.8℃。谱图数据为1hnmr(400mhz,cdcl3)δ:7.17-7.14(m,5h),6.98-6.95(m,3h),3.53-3.46(m,1h),3.03(dd,j=13.5,6.1hz,1h),2.90(d,j=3.4hz,6h),2.79(dd,j=13.5,8.8hz,1h),2.61(d,j=6.9hz,2h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:170.8,146.0,138.0,134.1,131.9,130.5,129.6,128.2,127.4,126.7,126.3,43.5,41.7,38.7,37.2,35.5ppm.hrms:calcdforc18h20cl2no[m+h]+336.0922,found336.0924.
实施例13
实施例13所得产物的收率是(28.6mg,85%yield),产物的氢谱和碳谱核磁共振谱图分别为图13a和13b,熔点mp:108.4-110.0℃。谱图数据为1hnmr(400mhz,cdcl3)δ:7.20(d,j=8.4hz,2h),7.15(d,j=8.3hz,2h),7.04(d,j=8.4hz,2h),6.94(d,j=8.3hz,2h),3.52-3.45(m,1h),3.03(dd,j=13.5,6.0hz,1h),2.88(d,j=2.5hz,6h),2.77(dd,j=13.5,8.9hz,1h),2.60(d,j=7.0hz,2h).ppm.13c{1h}nmr(101mhz,cdcl3)δ:170.9,142.3,138.0,132.0,131.8,130.5,129.0,128.4,128.2,43.2,41.8,38.8,37.1,35.41ppm.hrms:calcdforc18h20cl2no[m+h]+336.0922,found336.0921.
实施例14
实施例14所得产物的收率是(28.8mg,82%yield),产物的氢谱和碳谱核磁共振谱图分别为图14a和14b,谱图数据为1hnmr(400mhz,cdcl3)δ:8.16(d,j=8.5hz,1h),7.86-7.74(m,1h),7.69(d,j=8.1hz,1h),7.50-7.37(m,3h),7.30(d,j=7.1hz,1h),7.10-7.07(m,2h),7.00-6.98(m,2h),4.51(s,1h),3.18(dd,j=13.7,6.1hz,1h),3.09(dd,j=13.7,8.0hz,1h),2.82(d,j=22.5hz,6h),2.71(d,j=6.9hz,2h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.2,139.9,138.3,133.9,131.6,131.5,130.5,128.8,128.0,126.9,126.0,125.4,125.1,123.1,40.5,38.5,37.0,35.4ppm.hrms:calcdforc22h23clno[m+h]+352.1468,found352.1469.
实施例15
实施例15所得产物的收率是(26.6mg,83%yield),产物的氢谱和碳谱核磁共振谱图分别为图15a和15b,熔点mp:97.4-99.6℃。谱图数据为1hnmr(400mhz,cdcl3)δ:7.22-7.13(m,3h),6.98-6.94(m,2h),6.89-6.82(m,3h),3.55-3.48(m,1h),3.04(dd,j=13.5,6.0hz,1h),2.90(d,j=2.9hz,6h),2.79(dd,j=13.5,8.9hz,1h),2.62-2.60(m,2h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:170.9,162.8(q,j1c-f=246.7hz),146.5(q,j5c-f=6.7hz),138.0,131.8,130.5,129.7(q,j4c-f=8.4hz),128.2,123.6(q,j6c-f=2.7hz),114.3(q,j2c-f=21.2hz),113.3(q,j3c-f=21.1hz),43.5(q,j7c-f=1.7hz),41.7,38.8,37.2,35.5ppm.hrms:calcdforc18h20clfno[m+h]+320.1217,found320.1212.
实施例16
实施例16所得产物的收率是(20.8mg,71%yield),产物的氢谱和碳谱核磁共振谱图分别为图16a和16b,谱图数据为1hnmr(400mhz,cdcl3)δ:7.25-7.09(m,8h),7.08-7.05(m,2h),3.59-3.52(m,1h),3.36-3.20(m,2h),3.18-3.09(m,1h),3.04(dd,j=13.4,6.7hz,1h),2.91(dd,j=13.4,8.3hz,2h),2.59(d,j=7.1hz,2h),1.04-0.98(m,6h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:170.5,144.1,140.0,129.2,128.2,128.0,127.7,126.2,125.8,44.0,42.3,41.8,40.1,38.8,14.3,13.0ppm.hrms:calcdforc20h26no[m+h]+296.2014,found296.2017.
实施例17
实施例17所得产物的收率是(20.5mg,69%yield),产物的氢谱和碳谱核磁共振谱图分别为图17a和17b,谱图数据为1hnmr(400mhz,cdcl3)δ:7.27-7.22(m,2h),7.19-7.13(m,3h),6.77(s,1h),6.70(s,2h),3.55-3.47(m,1h),2.94-2.85(m,2h),2.82(d,j=10.0hz,6h),2.60(dd,j=7.0,3.5hz,2h),2.21(s,6h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.5,144.5,139.8,137.4,128.2,127.6,127.5,127.1,126.2,43.7,42.4,38.7,37.1,35.3,21.2ppm.hrms:calcdforc20h26no[m+h]+296.2014,found296.2019.
实施例18
实施例18所得产物的收率是(20.5mg,69%yield),产物的氢谱和碳谱核磁共振谱图分别为图18a和18b,熔点mp:99.0-100.8℃。谱图数据为1hnmr(400mhz,cdcl3)δ:7.25-7.20(m,2h),7.18-7.08(m,5h),6.96-6.93(m,2h),3.51-3.44(m,1h),3.04(dd,j=13.4,6.0hz,1h),2.86-2.79(m,7h),2.62-2.60(m,2h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.3,143.7,138.5,131.6,130.5,128.3,128.1,127.6,126.4,43.8,41.7,39.0,37.2,35.4ppm.hrms:calcdforc18h21clno[m+h]+302.1312,found302.1313.
实施例19
实施例19所得产物的收率是(29.5mg,97%yield),产物的氢谱和碳谱核磁共振谱图分别为图19a和19b,熔点mp:70.2-73.2℃。谱图数据为1hnmr(400mhz,cdcl3)δ:171.5,144.5,138.1,136.4,130.1,130.0,128.2,127.5,126.3,126.0,125.4,42.6,40.1,38.9,37.2,35.4,19.4ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.5,144.5,138.1,136.4,130.1,130.0,128.2,127.5,126.3,126.0,125.4,42.6,40.1,38.9,37.2,35.4,19.4ppm.hrms:calcdforc19h24no[m+h]+282.1858,found282.1857.
实施例20
实施例20所得产物的收率是(20.5mg,73%yield),产物的氢谱和碳谱核磁共振谱图分别为图20a和20b,熔点mp:77.6-81.0℃。谱图数据为1hnmr(400mhz,cdcl3)δ:7.26-7.22(m,2h),7.18-7.14(m,3h),7.08-7.05(m,1h),6.95-6.92(m,2h),6.85-6.83(d,j=7.4hz,1h),3.56-3.49(m,1h),2.98(dd,j=13.4,7.0hz,1h),2.90-2.82(m,7h),2.65-2.58(m,2h),2.26(s,3h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.5,144.3,139.8,137.5,130.0,128.2,127.8,127.6,126.6,126.2,126.2,43.8,42.4,38.8,37.1,35.3,21.3ppm.hrms:calcdforc19h24no[m+h]+282.1858,found282.1862.
实施例21
实施例21所得产物的收率是(12.1mg,43%yield),产物的氢谱和碳谱核磁共振谱图分别为图21a和21b,熔点mp:88.2-89.9℃。谱图数据为1hnmr(400mhz,cdcl3)δ:7.26-7.22(m,2h),7.17-7.14(m,3h),7.00-6.94(m,4h),3.54-3.47(m,1h),2.98(dd,j=13.5,6.9hz,1h),2.90-2.87(m,1h),2.83(d,j=9.5hz,6h),2.61(dd,j=7.0,1.9hz,2h),2.26(s,3h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.5,144.3,136.8,135.2,129.0,128.7,128.2,127.6,126.2,43.8,42.0,38.9,37.1,35.3,21.0.hrms:calcdforc19h24no[m+h]+282.1858,found282.1863.
实施例22
实施例22所得产物的收率是(29mg,84%yield),产物的氢谱和碳谱核磁共振谱图分别为图22a和22b,熔点mp:112.4-115.2℃。谱图数据为1hnmr(400mhz,cdcl3)δ:7.48(dd,j=7.9,1.3hz,1h),7.27-7.15(m,5h),7.12-7.08(m,1h),7.05-6.97(m,2h),3.66-3.59(m,1h),3.21(dd,j=13.7,7.3hz,1h),3.02(dd,j=13.7,7.9hz,1h),2.84(d,j=10.3hz,6h),2.76(dd,j=15.1,8.0hz,1h),2.63(dd,j=15.1,6.3hz,1h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.3,143.8,139.3,132.7,131.4,128.3,127.7,127.6,127.0,126.4,124.9,42.3,42.2,38.9,37.3,35.3.hrms:calcdforc18h21brno[m+h]+346.0807,found346.0811.
实施例23
实施例23所得产物的收率是(28mg,93%yield),产物的氢谱和碳谱核磁共振谱图分别为图23a和23b,熔点mp:97.2-99.1℃。谱图数据为1hnmr(400mhz,cdcl3)δ:7.30-7.14(m,6h),7.10-7.04(m,3h),3.66-3.58(m,1h),3.20(dd,j=13.7,7.3hz,1h),3.02(dd,j=13.7,7.9hz,1h),2.84(d,j=7.5hz,6h),2.74(dd,j=15.1,7.9hz,1h),2.62(dd,j=15.1,6.3hz,1h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.3,143.9,137.6,134.2,131.3,129.3,128.3,127.5,127.4,126.4,126.4,42.3,39.6,38.9,37.2,35.3ppm.hrms:calcdforc18h21clno[m+h]+302.1312,found302.1312.
实施例24
实施例24所得产物的收率是(22.1mg,56%yield),产物的氢谱和碳谱核磁共振谱图分别为图24a和24b,熔点mp:112.6-114.8℃。谱图数据为1hnmr(400mhz,cdcl3)δ:7.78-7.76(m,1h),7.27-7.12(m,6h),7.04-7.01(m,1h),6.84-6.80(m,1h),3.64-3.56(m,1h),3.18(dd,j=13.7,7.4hz,1h),3.02(dd,j=13.7,7.9hz,1h),2.85(d,j=13.0hz,6h),2.77(dd,j=15.0,8.1hz,1h),2.63(dd,j=15.0,6.1hz,1h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.2,143.6,142.5,139.4,130.5,128.3,127.9,127.8,127.6,126.4,101.3,46.6,42.5,38.8,37.3,35.3ppm.hrms:calcdforc18h21ino[m+h]+394.0668,found394.0673.
实施例25
实施例25所得产物的收率是(29mg,91%yield),产物的氢谱和碳谱核磁共振谱图分别为图25a和25b,熔点mp:108.4-109.6℃。谱图数据为1hnmr(400mhz,cdcl3)δ:8.20(d,j=8.4hz,1h),7.74-7.72(m,1h),7.56(d,j=8.2hz,1h),7.48-7.44(m,1h),7.39-7.35(m,1h),7.16-7.11(m,3h),7.08-6.02(m,3h),6.91(d,j=6.9hz,1h),3.67-3.54(m,2h),3.12(dd,j=13.4,8.0hz,1h),2.78(d,j=18.7hz,6h),2.65(d,j=6.8hz,2h)ppm.13c{1h}nmr(101mhz,cdcl3)δ:171.5,144.5,136.0,133.8,132.2,128.5,128.2,127.5,127.4,126.7,126.3,125.9,125.3,125.0,124.3,42.7,39.9,39.4,37.2,35.4ppm.hrms:calcdforc22h24no[m+h]+318.1858,found318.1861.
- 还没有人留言评论。精彩留言会获得点赞!