一种吡唑烷酮并苯并1,3-氧氮杂卓类化合物的合成方法
本发明属于有机合成技术领域,具体涉及一种吡唑烷酮并苯并1,3-氧氮杂卓类化合物的合成方法。
背景技术:
苯并氧氮杂卓是一类重要的同时含氮、氧两种杂原子的稠杂环化合物,该类化合物不仅在自然界中广泛存在,而且许多都具有抗惊厥、抗肿瘤、抗抑郁、抗焦虑、抑菌等生理及药理活性。
目前,该类化合物主要利用双官能团化苯(特别是双卤代苯或单卤代苯等)与其它组分化合物的缩合反应来合成。这些方法尽管相对可靠,但所用原料需要经多步反应制得,成本高且不易得到。另外,反应结束后卤素等原子的丢失也使得反应的原子经济性较低,且产生大量副产物。
因此,研究并开发从简单易得的原料出发,经过简洁的操作来合成苯并1,3-氧氮杂卓类化合物的高效绿色新方法,具有十分重要的理论意义和应用前景。
技术实现要素:
本发明解决的技术问题是提供了一种吡唑烷酮并苯并1,3-氧氮杂卓类化合物的合成方法,该方法通过1-芳基吡唑烷酮类化合物和重氮萘酮类化合物之间发生串联反应,高效地、区域选择性地合成了吡唑烷酮并苯并1,3-氧氮杂卓类化合物,具有原料简单易得、操作简便、条件温和、选择性好及底物适用范围广等优点。
本发明为解决上述技术问题采用如下技术方案,一种吡唑烷酮并苯并1,3-氧氮杂卓类化合物的合成方法,包括如下操作:以1-芳基吡唑烷酮1和重氮萘酮类化合物2为原料,在催化剂、氧化剂和添加剂存在下,有机溶剂中升温反应得到吡唑烷酮并苯并1,3-氧氮杂卓类化合物3,反应方程式为:
其中r1为氢、卤素、三氟甲基、氰基、c1-4烷基、c1-4烷氧基或苄氧基,r2为氢、卤素、氰基、乙酰基、苄基、c1-4烷基、c1-4烷氧基、苯基或取代苯基,取代苯基的取代基为卤素、三氟甲基、氰基、乙酰基、c1-4烷基或c1-4烷氧基。
进一步地,在上述技术方案中,所述反应溶剂为起到溶解原料的作用,优选1,2-二氯乙烷、二氯甲烷、乙腈或甲苯。
进一步地,在上述技术方案中,所述氧化剂为醋酸银、过氧化银、三氟乙酸银、碳酸银、氧化铜、醋酸铜或硫酸铜。
进一步地,在上述技术方案中,所述添加剂为醋酸、三甲基乙酸、2,4,6-三甲基苯甲酸或1-金刚烷甲酸。
进一步地,在上述技术方案中,所述催化剂为二氯(五甲基环戊二烯基)合铑(iii)二聚体([rhcp*cl2]2)、五甲基环戊二烯基醋酸铑(iii)(rhcp*(oac)2)、(五甲基环戊二烯基)二氯化铱(iii)二聚体([ircp*cl2]2)或二(六氟锑酸)三乙腈(五甲基环戊二烯基)铑(iii)(rhcp*(mecn)3(sbf6)2)。
进一步地,在上述技术方案中,所述的1-芳基吡唑烷酮1、重氮萘酮类化合物2、氧化剂、添加剂和催化剂的投料摩尔比为1:1-1.5:1.0-2.5:0.5-3.0:0.025。
进一步地,在上述技术方案中,所述反应温度为60-120℃。
进一步地,在上述技术方案中,反应中额外添加分子筛(例如
进一步地,在上述技术方案中,反应在氩气氛围下进行。
发明有益效果:
本发明与现有技术相比具有以下优点:(1)合成过程简单、高效,通过1-芳基吡唑烷酮和重氮萘酮类化合物的一锅串联反应,即可高选择性地合成吡唑烷酮并苯并1,3-氧氮杂卓类化合物;(2)原料价廉易得;反应条件温和,操作简便;(3)反应的原子经济性高,符合绿色化学的要求。
说明书附图
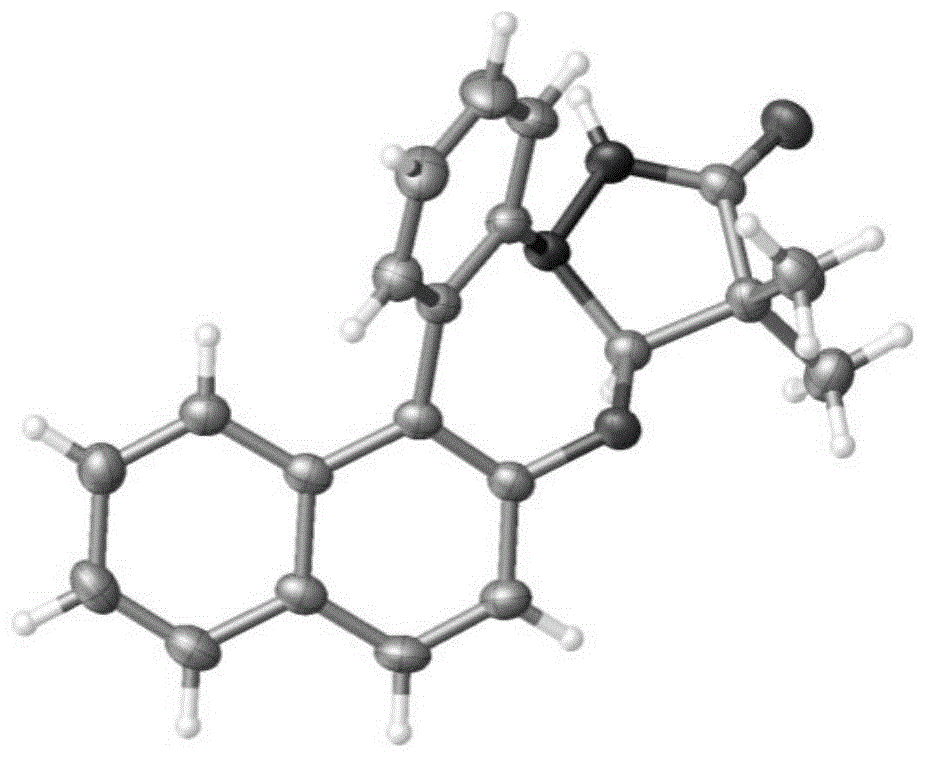
图1为实施例2中化合物3a的x-射线单晶衍射图。
具体实施方式
以下通过实施例对本发明的上述内容做进一步详细说明,但不应该将此理解为本发明上述主题的范围仅限于以下的实施例,凡基于本发明上述内容实现的技术均属于本发明的范围。
实施例1
在氩气氛围下,向15ml反应瓶中依次加入1a、2a、有机溶剂、氧化剂、添加剂和催化剂,盖上塞子密封,将其置于油浴中升温搅拌反应。待反应结束后,冷却至室温,萃取,干燥,旋干,过硅胶柱分离(石油醚/乙酸乙酯=3/1)得白色固体产物3a。反应方程式表示为:
通过改变反应溶剂、氧化剂、添加剂、催化剂、反应温度和反应物之间当量比等反应条件,实验结果见表1。
表1不同反应条件下3a的合成a
实施例2
氩气氛围下,向15ml反应瓶中依次加入1a(57.0mg,0.3mmol)、2a(76.5mg,0.45mmol)、乙腈(3ml)、[rhcp*cl2]2(4.7mg,0.0075mmol)、醋酸银(100.1mg,0.6mmol)和2,4,6-三甲基苯甲酸(98.5mg,0.6mmol),盖上塞子密封,将其置于100℃油浴中搅拌反应12小时,待反应结束后,冷却至室温,萃取,干燥,旋干,过硅胶柱分离(石油醚/乙酸乙酯=3/1)得白色固体产物3a(74.3mg,75%)。x-射线单晶衍射图为图1。mp244.8-245.7℃.1hnmr(600mhz,cdcl3):δ8.13-8.12(m,1h),8.10(s,1h),7.91-7.89(m,1h),7.86(d,j=9.0hz,1h),7.57(dd,j1=7.8hz,j2=1.8hz,1h),7.50-7.47(m,3h),7.42-7.39(m,1h),7.28(d,j=9.0hz,1h),7.23(td,j1=7.8hz,j2=1.2hz,1h),5.52(s,1h),1.29(s,3h),1.13(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.3,151.2,146.5,132.6,132.0,131.5,130.2,128.8,128.6,128.4,126.9,125.6,125.4,124.6,122.8,119.7,115.9,109.2,43.6,21.4,18.7.hrms(esi)m/z:[m+na]+calcdforc21h18n2nao2353.1260;found353.1256.
实施例3
依照实施例2的方法和步骤,通过改变反应物1和2,合成出各种吡唑烷酮并苯并1,3-氧氮杂卓类化合物3,具体结果见表2。
表2各种吡唑烷酮并苯并1,3-氧氮杂卓类化合物3的合成a,b
代表性产物表征数据如下:
8,8,14-trimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3b)
whitesolid(68.1mg,66%),mp241.2-242.2℃.1hnmr(400mhz,cdcl3):δ8.14-8.12(m,1h),8.08(s,1h),7.89-7.86(m,1h),7.82(d,j=8.8hz,1h),7.51-7.45(m,2h),7.37(d,j=1.2hz,1h),7.34(d,j=8.4hz,1h),7.25(s,1h),7.19(dd,j1=8.4hz,j2=1.6hz,1h),5.45(s,1h),2.39(s,3h),1.27(s,3h),1.12(s,3h).13c{1h}nmr(100mhz,cdcl3):δ178.3,151.2,144.1,132.6,132.5,132.2,131.5,130.1,129.4,128.6,128.5,126.8,125.7,125.4,124.4,119.8,115.9,108.9,43.5,21.5,20.8,18.8.hrms(esi)m/z:[m+na]+calcdforc22h20n2nao2367.1417;found367.1405.
14-ethyl-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3c)
lightyellowsolid(64.5mg,60%),mp195.6-196.5℃.1hnmr(600mhz,cdcl3):δ8.15-8.13(m,1h),7.90(dd,j1=7.2hz,j2=3.0hz,1h),7.87(d,j=9.0hz,1h),7.52-7.47(m,3h),7.41(d,j=1.8hz,1h),7.39(d,j=8.4hz,1h),7.31(d,j=8.4hz,1h),7.23(dd,j1=8.4hz,j2=2.4hz,1h),5.54(s,1h),2.70(q,j=7.8hz,2h),1.31(s,3h),1.29(t,j=7.8hz,3h),1.15(s,3h).13c{1h}nmr(100mhz,cdcl3):δ178.2,151.2,144.2,138.7,132.6,131.54,131.46,130.1,128.59,128.57,128.2,126.9,125.6,125.4,124.5,119.8,115.8,109.0,43.5,28.2,21.5,18.8,15.7.hrms(esi)m/z:[m+h]+calcdforc23h23n2o2359.1754;found359.1750.
14-methoxy-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3d)
whitesolid(76.7mg,71%),mp221.7-222.7℃.1hnmr(600mhz,cdcl3):δ8.40(s,1h),8.17-8.16(m,1h),7.87-7.86(m,1h),7.79(d,j=9.0hz,1h),7.49-7.45(m,2h),7.34(d,j=9.0hz,1h),7.22(d,j=9.0hz,1h),7.13(d,j=2.4hz,1h),6.93(dd,j1=9.0hz,j2=2.4hz,1h),5.35(s,1h),3.80(s,3h),1.24(s,3h),1.10(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.4,155.4,151.3,139.9,132.5,131.4,130.3,128.6,128.2,127.0,125.7,125.5,125.4,119.9,117.9,116.9,113.7,108.6,55.7,43.3,21.6,18.9.hrms(esi)m/z:[m+na]+calcdforc22h20n2nao3383.1366;found383.1370.
14-(benzyloxy)-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3e)
whitesolid(85.1mg,65%),mp213.7-214.4℃.1hnmr(600mhz,cdcl3):δ7.99(d,j=8.4hz,1h),7.89-7.86(m,2h),7.53(s,1h),7.47-7.38(m,7h),7.35(t,j=7.2hz,1h),7.30(d,j=9.0hz,1h),7.20(d,j=2.4hz,1h),7.05(dd,j1=8.4hz,j2=1.8hz,1h),5.50(s,1h),5.11(d,j=12.0hz,1h),5.07(d,j=12.0hz,1h),1.31(s,3h),1.14(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.4,154.5,151.2,140.2,137.0,132.5,131.3,130.4,128.7,128.6,128.1,128.0,127.5,127.1,125.7,125.43,125.41,119.8,118.6,116.9,115.3,108.9,70.4,43.3,21.6,18.8.hrms(esi)m/z:[m+h]+calcdforc28h25n2o3437.1860;found437.1863.
14-fluoro-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3f)
whitesolid(68.9mg,66%),mp239.2-240.1℃.1hnmr(600mhz,cdcl3):δ8.11(d,j=7.8hz,1h),7.95(s,1h),7.91-7.89(m,2h),7.54-7.49(m,2h),7.44-7.42(m,1h),7.31-7.30(m,2h),7.11(t,j=7.2hz,1h),5.51(s,1h),1.30(s,3h),1.13(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.5,158.8(d,1jc-f=240.6hz),151.4,142.6(d,4jc-f=2.1hz),132.5,131.2,130.9,128.7,127.3,127.2,126.2(d,3jc-f=7.7hz),125.7,125.1,119.7,118.6(d,2jc-f=24.0hz),117.1(d,3jc-f=8.7hz),115.2(d,2jc-f=21.9hz),109.1,43.5,21.5,18.8.19fnmr(565mhz,cdcl3):δ-126.99–-127.02(m).hrms(esi)m/z:[m+h]+calcdforc21h18fn2o2349.1347;found349.1356.
14-chloro-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3g)
whitesolid(84.1mg,77%),mp259.2-260.1℃.1hnmr(600mhz,cdcl3):δ8.09(d,j=8.4hz,1h),7.92(d,j=8.4hz,2h),7.56-7.50(m,4h),7.44(d,j=9.0hz,1h),7.37(dd,j1=9.0hz,j2=2.4hz,1h),7.32(d,j=9.0hz,1h),5.56(s,1h),1.32(s,3h),1.14(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.3,151.4,145.2,132.5,131.5,131.2,130.9,128.7,128.5,128.2,127.4,127.0,126.2,125.7,125.0,119.7,117.2,109.3,43.6,21.3,18.8.hrms(esi)m/z:[m+h]+calcdforc21h18cln2o2365.1051;found365.1050.
14-bromo-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3h)
whitesolid(86.9mg,71%),mp262.7-263.4℃.1hnmr(400mhz,cdcl3):δ8.09(d,j=8.4hz,1h),7.91(d,j=8.8hz,2h),7.69(d,j=1.6hz,1h),7.57-7.51(m,4h),7.38(d,j=8.8hz,1h),7.31(d,j=8.8hz,1h),5.56(s,1h),1.32(s,3h),1.14(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.2,151.4,145.8,134.3,132.5,131.5,131.2,131.0,128.7,127.5,126.9,126.6,125.7,125.0,119.6,117.6,115.6,109.3,43.6,21.3,18.8.hrms(esi)m/z:[m+h]+calcdforc21h18brn2o2409.0546;found409.0548.
8,8-dimethyl-14-(trifluoromethyl)-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3i)
yellowsolid(69.3mg,58%),mp206.1-207.1℃.1hnmr(600mhz,cdcl3):δ8.11(s,1h),8.01(d,j=8.4hz,1h),7.93(d,j=8.4hz,2h),7.82(d,j=1.8hz,1h),7.66(dd,j1=9.0hz,j2=1.8hz,1h),7.59(d,j=8.4hz,1h),7.56-7.51(m,2h),7.32(d,j=9.0hz,1h),5.61(s,1h),1.32(s,3h),1.14(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.4,151.6,149.5,132.6,131.3,131.1,129.0(q,3jc-f=3.3hz),128.8,127.6,127.0,125.79(q,3jc-f=3.3hz),125.78,124.9(q,2jc-f=32.9hz),124.8,124.3(q,1jc-f=270.2hz),119.6,116.0,109.6,43.9,21.0,18.7.19fnmr(376mhz,cdcl3):δ-61.75(s).hrms(esi)m/z:[m+h]+calcdforc22h18f3n2o2399.1315;found399.1313.
8,8-dimethyl-9-oxo-7a,8,9,10-tetrahydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepine-14-carbonitrile(3j)
yellowsolid(66.1mg,62%),mp266.1-266.7℃.1hnmr(400mhz,cdcl3):δ8.41(s,1h),7.98-7.92(m,3h),7.83(d,j=2.0hz,1h),7.65(dd,j1=8.8hz,j2=2.0hz,1h),7.59-7.52(m,3h),7.31(d,j=8.8hz,1h),5.63(s,1h),1.31(s,3h),1.13(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.3,151.3,147.8,134.7,133.0,132.6,131.4,130.6,128.7,127.4,127.1,125.6,125.2,123.0,122.9,119.6,116.2,109.3,43.7,21.2,18.8.hrms(esi)m/z:[m+na]+calcdforc22h17n3nao2378.1213;found378.1203.
13-chloro-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3k)
whitesolid(78.6mg,72%),mp213.4-214.2℃.1hnmr(400mhz,dmso-d6):δ10.3(s,1h),8.12-8.08(m,2h),8.02-8.00(m,1h),7.63-7.56(m,3h),7.49(d,j=8.8hz,1h),7.37(d,j=2.0hz,1h),7.31(dd,j1=8.0hz,j2=1.6hz,1h),5.70(s,1h),1.24(s,3h),1.01(s,3h).13c{1h}nmr(150mhz,dmso-d6):δ176.3,151.7,148.9,133.8,133.6,132.6,131.2,131.1,129.3,127.9,127.2,126.1,125.0,122.8,122.5,120.6,116.1,108.8,43.6,21.2,19.3.hrms(esi)m/z:[m+na]+calcdforc21h17cln2nao2387.0871;found387.0879.
13-bromo-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3l)
whitesolid(80.8mg,66%),mp265.4-266.4℃.1hnmr(400mhz,cdcl3):δ8.05-8.04(m,1h),7.90(d,j=8.4hz,2h),7.65(brs,2h),7.51-7.49(m,2h),7.42(d,j=8.4hz,1h),7.35(d,j=8.4hz,1h),7.31(d,j=8.8hz,1h),5.57(s,1h),1.33(s,3h),1.18(s,3h).13c{1h}nmr(100mhz,cdcl3):δ178.3,151.3,147.8,133.2,132.6,131.3,130.7,128.7,127.4,127.2,125.9,125.6,125.2,123.5,122.7,119.7,119.0,109.4,43.7,21.2,18.8.hrms(esi)m/z:[m+h]+calcdforc21h18brn2o2409.0546;found409.0551.
13-fluoro-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3m)
whitesolid(63.7mg,61%),mp216.8-217.7℃.1hnmr(400mhz,cdcl3):δ8.10-8.06(m,2h),7.92-7.89(m,2h),7.51-7.49(m,2h),7.31(d,j=8.8hz,2h),7.15-7.11(m,2h),5.38(s,1h),1.34(s,3h),1.27(s,3h).13c{1h}nmr(150mhz,cdcl3):δ176.3,154.8(d,1jc-f=250.0hz),151.6,132.8(d,4jc-f=2.1hz),132.6,131.5,130.8,128.6,128.5,128.4,128.0(d,3jc-f=3.3hz),127.6,127.1,125.5(d,2jc-f=12.2hz),123.2(d,3jc-f=8.7hz),119.6,117.5(d,2jc-f=23.0hz),109.3,44.0,21.5,17.9.19fnmr(376mhz,cdcl3):δ-126.91–-126.96(m).hrms(esi)m/z:[m+h]+calcdforc21h18fn2o2349.1347;found349.1338.
3-ethyl-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3n)
whitesolid(73.1mg,68%),mp245.4-246.2℃.1hnmr(400mhz,cdcl3):δ8.05-8.02(m,2h),7.77(d,j=8.8hz,1h),7.67(s,1h),7.55(d,j=7.6hz,1h),7.46(d,j=8.0hz,1h),7.40-7.34(m,2h),7.25-7.19(m,2h),5.48(s,1h),2.81(q,j=7.2hz,2h),1.33(t,j=7.2hz,3h),1.27(s,3h),1.12(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.3,150.7,146.5,141.4,132.9,131.9,129.9,129.7,128.7,128.1,126.2,125.5,124.8,122.8,119.6,115.8,109.2,43.6,28.8,21.4,18.7,15.5.hrms(esi)m/z:[m+na]+calcdforc23h22n2nao2381.1573;found381.1571.
8,8-dimethyl-3-propyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3o)
whitesolid(73.7mg,66%),mp217.8-218.1℃.1hnmr(400mhz,cdcl3):δ8.03(d,j=8.8hz,1h),7.80-7.78(m,2h),7.66(s,1h),7.57(d,j=7.6hz,1h),7.47(d,j=8.0hz,1h),7.41-7.33(m,2h),7.26-7.20(m,2h),5.52(s,1h),2.75(t,j=7.6hz,2h),1.78-1.69(m,2h),1.29(s,3h),1.13(s,3h),0.98(t,j=7.2hz,3h).13c{1h}nmr(150mhz,cdcl3):δ178.3,150.6,146.5,139.8,132.8,131.9,129.9,129.7,128.7,128.5,128.1,127.1,125.4,124.8,122.8,119.6,115.8,109.2,43.6,37.9,24.4,21.4,18.7,13.9.hrms(esi)m/z:[m+na]+calcdforc24h24n2nao2395.1730;found395.1719.
8,8-dimethyl-3-phenyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3p)
yellowsolid(74.3mg,61%),mp259.6-260.2℃.1hnmr(600mhz,cdcl3):δ8.10(d,j=8.4hz,1h),8.02(d,j=1.2hz,1h),7.84(d,j=9.0hz,1h),7.67-7.64(m,4h),7.53-7.52(m,1h),7.43-7.41(m,3h),7.36-7.31(m,2h),7.25(d,j=9.0hz,1h),7.19-7.17(m,1h),5.49(s,1h),1.24(s,3h),1.07(s,3h).13c{1h}nmr(100mhz,cdcl3):δ178.3,151.3,146.6,140.6,138.1,132.9,132.0,130.6,130.5,129.0,128.9,128.3,127.6,127.4,126.6,126.3,126.2,124.6,122.9,120.2,115.9,109.2,43.6,21.4,18.7.hrms(esi)m/z:[m+h]+calcdforc27h23n2o2407.1754;found407.1756.
3-(2-fluorophenyl)-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3q)
whitesolid(91.6mg,72%),mp251.2-252.1℃.1hnmr(400mhz,cdcl3):δ8.12(d,j=8.8hz,1h),8.01(s,1h),7.87(d,j=8.8hz,1h),7.63(dt,j1=8.8hz,j2=1.6hz,1h),7.55(dd,j1=7.6hz,j2=1.2hz,1h),7.49(td,j1=7.6hz,j2=1.6hz,1h),7.44(d,j=8.0hz,1h),7.38-7.34(m,1h),7.32-7.28(m,2h),7.21-7.18(m,2h),7.16-7.11(m,2h),5.53(s,1h),1.27(s,3h),1.09(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.1,160.0(d,1jc-f=246.2hz),151.5,146.6,133.0,132.6,132.0,130.9(d,4jc-f=3.3hz),130.7,130.6,129.3(d,3jc-f=7.7hz),128.9,128.7(d,4jc-f=2.3hz),128.6(d,2jc-f=13.1hz),128.4,128.1(d,4jc-f=3.3hz),125.7,124.56(d,3jc-f=9.9hz),124.55,123.0,120.2,116.3(d,2jc-f=23.0hz),115.9,109.4,43.6,21.4,18.7.19fnmr(376mhz,cdcl3):δ-117.69–-117.81(m).hrms(esi)m/z:[m+h]+calcdforc27h22fn2o2425.1660;found425.1661.
3-bromo-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3r)
whitesolid(77.1mg,63%),mp251.8-252.5℃.1hnmr(400mhz,cdcl3):δ8.06(s,1h),7.97(d,j=8.8hz,1h),7.90(s,1h),7.77(d,j=8.4hz,1h),7.56-7.42(m,4h),7.33-7.24(m,2h),5.54(s,1h),1.30(s,3h),1.13(s,3h).13c{1h}nmr(100mhz,cdcl3):δ178.2,151.4,146.6,133.7,131.9,130.5,130.2,130.0,129.2,129.1,128.9,127.4,124.1,123.0,121.0,119.6,116.0,109.2,43.6,21.4,18.7.hrms(esi)m/z:[m+h]+calcdforc21h18brn2o2409.0546;found409.0541.
8,8-dimethyl-9-oxo-7a,8,9,10-tetrahydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepine-3-carbonitrile(3s)
whitesolid(75.6mg,71%),mp261.4-262.3℃.1hnmr(400mhz,dmso-d6):δ10.20(s,1h),8.12-8.09(m,2h),8.05(d,j=8.8hz,1h),7.71-7.69(m,1h),7.52-7.47(m,3h),7.41(d,j=8.0hz,1h),7.27(t,j=7.2hz,1h),5.63(s,1h),1.20(s,3h),0.95(s,3h).13c{1h}nmr(150mhz,cdcl3):δ176.3,152.3,147.5,132.4,131.7,131.5,131.1,130.9,129.7,129.0,127.8,127.1,123.4,122.9,121.5,121.4,116.6,108.8,43.4,21.5,19.2.hrms(esi)m/z:[m+h]+calcdforc22h18n3o2356.1394;found356.1376.
3-acetyl-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3t)
whitesolid(78.2mg,70%),mp259.1-260.1℃.1hnmr(400mhz,cdcl3):δ8.45(d,j=1.2hz,1h),8.11(d,j=9.2hz,1h),7.96(d,j=8.4hz,2h),7.45(d,j=7.6hz,2h),7.39-7.31(m,3h),7.21-7.17(m,1h),5.54(s,1h),2.67(s,3h),1.27(s,3h),1.08(s,3h).13c{1h}nmr(150mhz,cdcl3):δ197.8,178.1,153.1,146.6,134.0,133.9,132.0,131.9,131.8,130.6,129.2,128.9,126.2,124.9,124.1,123.1,120.9,116.0,109.3,43.6,26.7,21.4,18.7.hrms(esi)m/z:[m+na]+calcdforc23h20n2nao3395.1366;found395.1371.
2-benzyl-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3u)
yellowsolid(78.2mg,62%),mp213.4-214.3℃.1hnmr(400mhz,cdcl3):δ7.94(s,1h),7.90(s,1h),7.83-7.79(m,2h),7.52-7.47(m,2h),7.41-7.37(m,1h),7.32-7.23(m,4h),7.21-7.16(m,4h),5.54(s,1h),4.08(s,2h),1.29(s,3h),1.13(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.3,151.4,146.4,140.7,139.9,131.8,131.6,131.2,129.9,128.9,128.74,128.71,128.5,127.9,127.1,126.2,124.9,124.7,122.8,119.1,115.9,109.1,43.6,42.3,21.4,18.7.hrms(esi)m/z:[m+h]+calcdforc28h25n2o2421.1911;found421.1910.
8,8-dimethyl-2-phenyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3v)
lightbrownsolid(64.6mg,53%),mp146.8-147.7℃.1hnmr(600mhz,cdcl3):δ8.33(d,j=0.6hz,1h),8.16(s,1h),7.97(d,j=9.0hz,1h),7.87(d,j=9.0hz,1h),7.76(dd,j1=8.4hz,j2=1.2hz,1h),7.64-7.61(m,3h),7.50-7.49(m,1h),7.45(t,j=7.8hz,2h),7.43-7.40(m,1h),7.36(t,j=7.2hz,1h),7.28(d,j=9.0hz,1h),7.24(td,j1=7.8hz,j2=1.2hz,1h),5.52(s,1h),1.29(s,3h),1.15(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.3,151.6,146.6,141.1,139.7,131.9,131.8,131.7,129.9,129.1,128.9,128.6,127.6,127.5,125.3,124.7,123.6,123.0,119.8,116.0,109.2,43.6,21.4,18.8.hrms(esi)m/z:[m+na]+calcdforc27h22n2nao2429.1573;found429.1574.
2-methoxy-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3w)
whitesolid(78.9mg,73%),mp261.1-262.1℃.1hnmr(600mhz,cdcl3):δ7.82-7.79(m,2h),7.64(dd,j1=7.8hz,j2=1.8hz,1h),7.53-7.51(m,1h),7.47(d,j=2.4hz,1h),7.43-7.40(m,1h),7.28(s,1h),7.24(td,j1=7.8hz,j2=1.2hz,1h),7.18(d,j=8.4hz,1h),7.15(dd,j1=9.0hz,j2=2.4hz,1h),5.58(s,1h),3.81(s,3h),1.33(s,3h),1.15(s,3h).13c{1h}nmr(100mhz,cdcl3):δ178.2,158.7,151.9,146.5,132.9,131.3,130.11,130.07,128.7,128.1,127.0,125.0,122.9,118.0,117.2,116.0,109.2,104.2,55.3,43.5,21.4,18.7.hrms(esi)m/z:[m+h]+calcdforc22h21n2o3361.1547;found361.1542.
2-ethoxy-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3x)
whitesolid(84.2mg,75%),mp217.8-218.7℃.1hnmr(400mhz,cdcl3):δ7.82(s,1h),7.79-7.77(m,2h),7.62(d,j=7.2hz,1h),7.49(d,j=8.0hz,1h),7.44(d,j=2.0hz,1h),7.40(t,j=7.2hz,1h),7.23(t,j=7.6hz,1h),7.14(d,j=8.8hz,2h),5.53(s,1h),4.10-4.02(m,1h),4.01-3.94(m,1h),1.42(t,j=7.2hz,3h),1.30(s,3h),1.13(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.4,158.0,151.9,146.5,132.9,131.3,130.1,130.0,128.7,128.0,127.0,125.0,122.8,118.3,117.1,116.0,109.1,105.0,63.5,43.6,21.4,18.7,14.8.hrms(esi)m/z:[m+h]+calcdforc23h23n2o3375.1703;found375.1694.
2-bromo-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3y)
whitesolid(67.3mg,55%),mp275.1-275.6℃.1hnmr(400mhz,dmso-d6):δ10.2(s,1h),8.15-8.11(m,2h),8.06(d,j=8.4hz,1h),7.71(d,j=8.4hz,1h),7.54-7.49(m,3h),7.43(d,j=8.0hz,1h),7.28(t,j=7.2hz,1h),5.66(s,1h),1.22(s,3h),0.97(s,3h).13c{1h}nmr(150mhz,dmso-d6):δ176.3,152.3,147.5,132.4,131.7,131.5,131.1,130.9,129.7,129.0,127.8,127.1,123.4,122.9,121.5,121.4,116.6,108.8,43.4,21.5,19.2.hrms(esi)m/z:[m+h]+calcdforc21h18brn2o2409.0546;found409.0543.
2-benzyl-8,8,14-trimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3z)
whitesolid(78.2mg,60%),mp184.9-185.7℃.1hnmr(400mhz,dmso-d6):δ7.92(s,2h),7.79(d,j=8.4hz,2h),7.35-7.32(m,2h),7.29-7.23(m,4h),7.21-7.16(m,4h),5.49(s,1h),4.07(s,2h),2.33(s,3h),1.27(s,3h),1.12(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.3,151.4,144.0,140.7,140.0,132.5,132.2,131.7,131.2,129.8,129.3,129.0,128.7,128.6,128.0,127.1,126.3,124.9,124.5,119.2,115.9,108.9,43.5,42.4,21.5,20.8,18.8.hrms(esi)m/z:[m+h]+calcdforc29h27n2o2435.2067;found435.2064.
2,14-dimethoxy-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3aa)
brownsolid(71.4mg,61%),mp228.3-229.2℃.1hnmr(400mhz,cdcl3):δ7.82-7.79(m,2h),7.54-7.52(m,2h),7.41(d,j=8.8hz,1h),7.23(d,j=2.8hz,1h),7.19-7.14(m,2h),6.96(dd,j1=8.8hz,j2=2.4hz,1h),5.51(s,1h),3.82(s,6h),1.31(s,3h),1.14(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.5,158.7,155.6,152.0,139.9,132.7,130.2,128.0,126.9,126.0,118.1,117.4,117.1,116.7,114.1,108.7,104.0,55.7,55.3,43.3,21.7,18.8.hrms(esi)m/z:[m+h]+calcdforc23h23n2o4391.1652;found391.1653.
2-ethoxy-14-methoxy-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3bb)
brownsolid(52.1mg,43%),mp125.6-126.5℃.1hnmr(400mhz,cdcl3):δ7.79(d,j=8.4hz,2h),7.53(s,1h),7.50(s,1h),7.40(d,j=8.8hz,1h),7.21(d,j=2.4hz,1h),7.16-7.14(m,2h),6.95(dd,j1=8.8hz,j2=2.8hz,1h),5.49(s,1h),4.08-3.98(m,2h),3.82(s,3h),1.43(t,j=6.8hz,3h),1.30(s,3h),1.13(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.4,158.1,155.6,152.0,139.9,132.8,130.15,130.12,128.0,126.8,126.1,118.4,117.2,117.0,116.8,114.1,108.7,104.8,63.5,55.7,43.3,21.7,18.8,14.8.hrms(esi)m/z:[m+h]+calcdforc24h25n2o4405.1809;found405.1807.
14-methoxy-8,8-dimethyl-2-phenyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3cc)
lightbrownsolid(77.2mg,59%),mp203.4-204.3℃.1hnmr(600mhz,cdcl3):δ8.38(s,1h),7.98(d,j=8.4hz,1h),7.91(d,j=8.4hz,1h),7.75(dd,j1=8.4hz,j2=1.8hz,1h),7.63-7.62(m,2h),7.46-7.41(m,3h),7.38-7.35(m,2h),7.32(d,j=8.4hz,1h),7.21(d,j=3.0hz,1h),6.97(dd,j1=9.0hz,j2=2.4hz,1h),5.52(s,1h),3.81(s,3h),1.32(s,3h),1.15(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.3,155.6,151.7,141.0,140.0,139.8,131.72,131.67,130.2,129.2,128.9,128.4,127.6,127.5,125.8,125.3,123.4,119.9,117.6,117.0,114.1,108.9,55.7,43.3,21.7,18.9.hrms(esi)m/z:[m+h]+calcdforc28h25n2o3437.1860;found437.1857.
3-acetyl-13-chloro-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3dd)
whitesolid(63.4mg,52%),mp262.5-263.2℃.1hnmr(600mhz,cdcl3):δ8.52(s,1h),8.10(d,j=9.0hz,1h),8.04(d,j=8.4hz,2h),7.53(s,2h),7.45(d,j=8.4hz,1h),7.41(d,j=8.4hz,1h),7.23(dd,j1=7.8hz,j2=1.8hz,1h),5.61(s,1h),2.74(s,3h),1.34(s,3h),1.18(s,3h).13c{1h}nmr(150mhz,cdcl3):δ197.7,178.1,153.2,147.8,135.2,134.2,133.8,133.0,132.2,131.8,130.6,127.9,125.8,125.2,123.1,122.4,120.8,116.4,109.3,43.8,26.7,21.2,18.8.hrms(esi)m/z:[m+na]+calcdforc23h19cln2nao3429.0976;found429.0971.
3-acetyl-13-bromo-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3ee)
yellowsolid(82.4mg,61%),mp232.3-233.6℃.1hnmr(400mhz,dmso-d6):δ10.3(s,1h),8.83(s,1h),8.33(d,j=9.2hz,1h),8.09-8.04(m,2h),7.60(d,j=8.8hz,1h),7.52(s,1h),7.49-7.47(m,2h),5.74(s,1h),2.74(s,3h),1.24(s,3h),1.01(s,3h).13c{1h}nmr(150mhz,dmso-d6):δ198.1,176.2,153.5,148.9,134.1,133.9,133.3,133.1,131.9,131.6,127.6,125.6,125.4,122.8,122.6,121.7,119.0,108.8,43.6,27.2,21.2,19.2.hrms(esi)m/z:[m+h]+calcdforc23h20brn2o3451.0652;found451.0645.
13-chloro-2-methoxy-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3ff)
whitesolid(73.3mg,62%),mp240.7-241.5℃.1hnmr(400mhz,cdcl3):δ8.38(s,1h),7.81-7.78(m,2h),7.55(d,j=8.0hz,1h),7.50(d,j=2.0hz,1h),7.38(d,j=2.0hz,1h),7.20(dd,j1=8.4hz,j2=2.0hz,1h),7.15(d,j=8.4hz,2h),5.55(s,1h),3.82(s,3h),1.32(s,3h),1.17(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.6,158.8,152.0,147.7,134.6,132.8,132.3,130.3,130.2,128.0,126.1,123.3,122.8,118.2,117.1,116.3,109.1,103.8,55.3,43.8,21.2,18.8.hrms(esi)m/z:[m+na]+calcdforc22h19cln2nao3417.0976;found417.0973.
13-chloro-2-ethoxy-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3gg)
whitesolid(79.6mg,65%),mp227.7-228.5℃.1hnmr(400mhz,cdcl3):δ7.96(s,1h),7.82-7.78(m,2h),7.54(d,j=8.0hz,1h),7.55(d,j=2.0hz,1h),7.36(d,j=2.0hz,1h),7.20(dd,j1=8.0hz,j2=2.0hz,1h),7.15(d,j=8.8hz,2h),5.57(s,1h),4.11-3.96(m,2h),1.44(t,j=6.8hz,3h),1.32(s,3h),1.17(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.5,158.2,152.0,147.7,134.5,132.8,132.3,130.3,130.1,128.0,126.0,123.4,122.8,118.5,117.0,116.3,109.2,104.6,63.5,43.7,21.2,18.8,14.7.hrms(esi)m/z:[m+na]+calcdforc23h21cln2nao3431.1133;found431.1135.
13-bromo-2-methoxy-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3hh)
whitesolid(86.7mg,66%),mp218.9-219.7℃.1hnmr(400mhz,cdcl3):δ8.17(s,1h),7.82-7.78(m,2h),7.65(s,1h),7.48(d,j=8.0hz,1h),7.37-7.34(m,2h),7.16(d,j=8.8hz,2h),5.56(s,1h),3.82(s,3h),1.32(s,3h),1.17(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.5,158.9,152.0,147.8,132.7,132.5,130.4,130.2,128.0,126.1,125.8,123.9,122.6,119.2,118.2,117.1,109.2,103.8,55.3,43.8,21.2,18.8.hrms(esi)m/z:[m+h]+calcdforc22h20brn2o3439.0652;found439.0650.
13-bromo-2-ethoxy-8,8-dimethyl-7a,8-dihydrobenzo[d]naphtho[1,2-f]pyrazolo[5,1-b][1,3]oxazepin-9(10h)-one(3ii)
yellowsolid(74.6mg,55%),mp229.8-228.5℃.1hnmr(600mhz,cdcl3):δ8.00(s,1h),7.81-7.78(m,2h),7.64(d,j=1.8hz,1h),7.47(d,j=8.4hz,1h),7.35-7.34(m,2h),7.16-7.14(m,2h),5.56(s,1h),4.08-4.04(m,1h),4.02-3.98(m,1h),1.44(t,j=7.2hz,3h),1.32(s,3h),1.17(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.5,158.2,151.9,147.8,132.8,132.6,130.4,130.1,128.0,126.0,125.8,123.9,122.5,119.1,118.5,117.0,109.2,104.6,63.6,43.8,21.2,18.8,14.7.hrms(esi)m/z:[m+h]+calcdforc23h22brn2o3453.0808;found453.0813.
实施例4
本发明所合成的产物吡唑烷酮并苯并1,3-氧氮杂卓类化合物3可以进行一系列反应,从而合成进一步的衍生物。例如:
在0℃下,向3a(66.0mg,0.2mmol)的dmf(0.5ml)溶液中添加氢化钠(5.8mg,0.24mmol)/dmf(1ml)溶液并搅拌15分钟,然后在室温条件下将混合物搅拌1小时。随后,将反应混合物冷却至0℃,向反应体系内滴加碘甲烷(15μl,0.24mmol),滴加完毕后,将混合物恢复至室温并持续搅拌30分钟。反应结束后,再次将混合物冷却至0℃,加入饱和氯化铵溶液淬灭反应,并用乙酸乙酯萃取,合并有机相,用无水硫酸钠干燥,过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=3/1)得到白色固体产物4(34.4mg,50%)。该化合物的表征数据如下:1hnmr(400mhz,cdcl3):δ8.20-8.18(m,1h),7.93-7.89(m,2h),7.61(d,j=7.6hz,1h),7.53-7.47(m,2h),7.43-7.39(m,1h),7.33(d,j=8.8hz,1h),7.26-7.23(m,1h),7.07(d,j=8.0hz,1h),5.52(s,1h),3.10(s,3h),1.32(s,3h),1.12(s,3h).13c{1h}nmr(100mhz,cdcl3):δ176.0,151.2,144.6,132.6,132.2,131.4,130.2,128.63,128.60,128.4,126.8,125.7,125.6,125.4,122.9,119.8,116.4,108.2,44.1,31.5,22.1,18.6.hrms(esi)m/z:[m+h]+calcdforc22h21n2o2345.1598;found345.1587.
向15ml反应瓶中依次加入3h(39.5mg,0.1mmol)、乙炔基苯(16.5μl,0.15mmol)、pph3(5.2mg,0.02mmol)、k3po4(25.5mg,0.12mmol)、pd(oac)2(1.1mg,0.005mmol)和dmso(1ml),并将所得混合物在80℃、氩气氛围下搅拌24小时。反应结束后,用乙酸乙酯萃取,合并有机相,用无水硫酸钠干燥,过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=3/1)得到白色固体产物5(33mg,77%)。该化合物的表征数据如下:1hnmr(400mhz,cdcl3):δ8.15(d,j=8.4hz,1h),7.92-7.89(m,2h),7.75(d,j=1.6hz,1h),7.68(s,1h),7.59(dd,j1=8.4hz,j2=1.6hz,1h),7.56-7.47(m,5h),7.35-7.30(m,4h),5.58(s,1h),1.32(s,3h),1.15(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.2,151.4,146.6,135.1,132.6,132.1,131.6,131.4,130.6,128.6,128.4,128.2,127.5,127.3,125.6,125.4,124.7,123.3,119.6,117.7,116.0,109.4,89.3,89.0,43.8,21.2,18.7.hrms(esi)m/z:[m+h]+calcdforc29h23n2o2431.1754;found431.1740.
向15ml反应管中依次加入3h(40.8mg,0.1mmol)、苯基硼酸(24.4mg,0.2mmol)、pph3(15.7mg,0.06mmol)、k2co3(55.3mg,0.4mmol)、pd(oac)2(2.2mg,0.01mmol)和1,4-二氧六环(1ml)。将所得混合物在氩气氛围中于80℃搅拌反应24小时。反应结束后,用乙酸乙酯萃取,合并有机相,用无水硫酸钠干燥,过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=3/1)得到白色固体产物6(26.9mg,66%)。该化合物的表征数据如下:1hnmr(600mhz,cdcl3):δ8.19-8.17(m,1h),7.93-7.89(m,2h),7.83(d,j=1.8hz,2h),7.65(dd,j1=8.4hz,j2=1.8hz,1h),7.62(d,j=7.2hz,2h)7.56(d,j=8.4hz,1h),7.51-7.48(m,2h),7.43(t,j=7.2hz,2h),7.35-7.32(m,2h),5.58(s,1h),1.33(s,3h),1.19(s,3h).13c{1h}nmr(150mhz,cdcl3):δ178.2,151.4,145.8,140.2,135.7,132.7,131.6,130.6,130.4,128.9,128.7,128.3,127.3,127.17,127.15,126.8,125.5,124.9,119.8,116.4,109.2,43.7,21.4,18.9.hrms(esi)m/z:[m+na]+calcdforc27h22n2nao2429.1573;found429.1583.
在0℃下,向3a(66.0mg,0.2mmol)的乙酸乙酯(2ml)和甲醇(0.2ml)混合溶液中,分批加入硼氢化钠(15.1mg,0.4mmol),然后将该混合物在0℃搅拌反应10小时。反应结束后,加入饱和碳酸氢钠溶液淬灭反应,用乙酸乙酯萃取。合并有机相,用无水硫酸钠干燥,过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=3/1)得到白色固体产物v(29.9mg,45%)。该化合物的表征数据如下:1hnmr(600mhz,cdcl3):δ7.85(t,j=9.0hz,2h),7.77(s,1h),7.59(d,j=7.8hz,1h),7.54(d,j=7.8hz,1h),7.46-7.43(m,1h),7.42-7.38(m,2h),7.37-7.35(m,1h),7.29(d,j=8.4hz,1h),7.23(t,j=7.2hz,1h),3.19(d,j=11.4hz,1h),2.94(d,j=11.4hz,1h),1.02(s,3h),0.99(s,3h).13c{1h}nmr(100mhz,cdcl3):δ179.6,150.6,149.9,135.0,133.3,130.0,129.8,129.2,128.3,126.7,126.1,124.7,124.2,123.6,119.6,119.5,116.2,67.5,40.4,24.1,22.8.hrms(esi)m/z:[m+na]+calcdforc21h20n2nao2355.1417;found355.1406.
以上实施例描述了本发明的基本原理、主要特征及优点。本行业的技术人员应该了解,本发明不受上述实施例的限制,上述实施例和说明书中描述的只是说明本发明的原理,在不脱离本发明原理的范围下,本发明还会有各种变化和改进,这些变化和改进均落入本发明保护的范围内。
- 还没有人留言评论。精彩留言会获得点赞!