一种吲哚并七元环化合物及其制备方法和用途与流程
本发明属于药物化学技术领域,具体涉及一种吲哚并七元环化合物及其制备方法和用途。
背景技术:
吲哚并七元环骨架广泛存在于天然产物、合成药物中,相关研究表明含有该骨架的化合物具有多种重要的生物活性和药物活性,对此类化合物的取代基修饰、结构类似物的衍生化以及进一步生物学活性再评价成为了研究热点。
如何简便的制备收率高的吲哚并七元环化合物是目前的研究难点。
技术实现要素:
本发明的目的在于提供一种吲哚并七元环化合物及其制备方法和用途。
本发明提供了一种式(ⅰ)所示吲哚并七元环化合物或其立体化学异构体、溶剂合物或药学上可接受的盐:
其中,r1独立选自苯基、取代的苯基、萘基、乙氧羰基、c5~c6的杂环基、c4~c6的烷基;
r2独立选自乙氧羰基、
r3独立选自h、卤素;
r4独立选自h、烷基;
环a为含1~3个双键的七元环;
x独立选自n、nts。
进一步地,r3所述卤素,优选的为br;
r4所述烷基,优选的为甲基;
所述环a,优选的为
本发明还提供了上述化合物的制备方法,它包括以下步骤:将底物7(1.0eq)和溴巴豆酸乙酯(3.0eq)加入反应试管。以乙腈作为溶剂,碳酸铯作催化剂的条件下,反应2小时,反应完成后浓缩纯化得到目标产物15;
制备化合物的技术路线如下所示:
进一步地,底物的制备方法如下:
(1)将对甲基苯磺酰氯(1.0eq)投入500ml的干燥圆底烧瓶中,另外量取异丙醇(30ml)加入作为溶剂,放入干净的搅拌子;另取100ml的烧杯,称取迭氮钠(1.0eq)加入其中,再加入水作为溶剂,使其溶解完全;将迭氮钠(nan3)溶液倒入正在搅拌的500ml的对甲基苯磺酰氯(tscl),约1小时后添加200ml水,继续反应1小时;反应完成后使用二氯甲烷萃取,收集有机层,干燥浓缩得到油状化合物tsn3;
(2)称取吲哚(2eq)和tsn3无溶剂反应,60℃回流4h,颜色呈现红褐色,4小时后将60℃改至30℃,反应过夜;反应完成后,冷却至室温,加入无水甲醇,摇晃溶液,使其充分析出固体,放置几分钟,使固体充分析出后,将其过滤,过滤后得到粉红色的固体吲哚亚胺5;
(3)将吲哚亚胺(1.0eq)加入到封管中,加入20ml二氯甲烷,依次加入醛(1.5eq),三氟化硼乙醚(3eq),溶液呈现红棕色,反应1h,黄色固体析出过滤得产物;
制备底物的技术路线如下所示:
r=苯环,取代苯环,杂环,支链烷烃,直链烷烃,
本发明还提供了所述的化合物的还原和氧化产物的制备方法,技术路线如下:
(1)吲哚并七元环衍生物的还原
a法还原:
b法还原:
(2)吲哚并七元环衍生物的氧化:
本发明还提供了所述吲哚并七元环衍生物或其立体化学异构体、溶剂合物或药学上可接受的盐在制备抗肿瘤药物中的用途。其中,所述药物是治疗乳腺癌的药物;所述药物是治疗黑色素瘤的药物。
本发明还提供了所述吲哚并七元环衍生物或其立体化学异构体、溶剂合物或药学上可接受的盐为活性成分,加上药学上可接受的辅料制备而成的制剂。
关于本发明的使用术语的定义:除非另有说明,本文中基团或者术语提供的初始定义适用于整篇说明书的该基团或者术语;对于本文没有具体定义的术语,应该根据公开内容和上下文,给出本领域技术人员能够给予它们的含义。
“取代”是指分子中的氢原子被其它不同的原子或分子所替换。
卤素为氟、氯、溴或碘。
本发明中,术语“药学上可接受的盐”指本发明化合物与酸或碱所形成的适合用作药物的盐。药学上可接受的盐包括无机盐和有机盐。一类优选的盐是本发明化合物与碱金属形成的盐。适合形成盐的碱金属包括但并不限于:锂、钠,钾、钙、镁等。
本发明提供了一类新的化合物,其具有良好的抗肿瘤效果,化合物的制备方法简便、反应温和、收率高,具有广阔的市场应用前景。
显然,根据本发明的上述内容,按照本领域的普通技术知识和惯用手段,在不脱离本发明上述基本技术思想前提下,还可以做出其它多种形式的修改、替换或变更。
附图说明
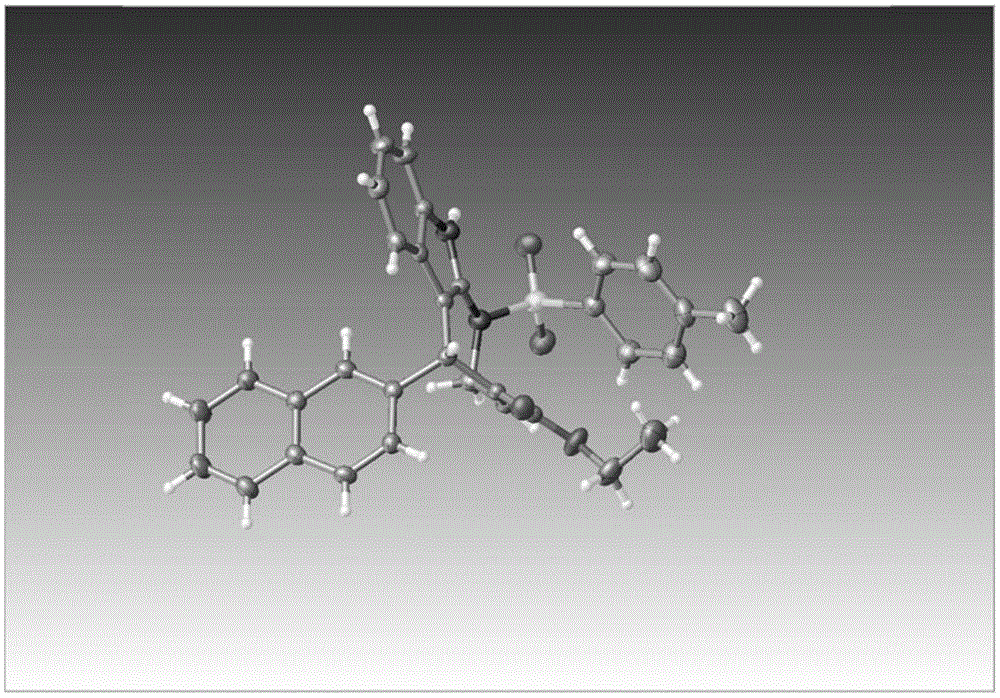
图1实施例15产物的单晶结构
图2实施例29产物的单晶结构
具体实施方式
以下通过实施例形式的具体实施方式,对本发明的上述内容再作进一步的详细说明。但不应将此理解为本发明上述主题的范围仅限于以下的实例。凡基于本发明上述内容所实现的技术均属于本发明的范围。
除特别标注外,本发明所用试剂和测试设备均为常规的市售试剂和设备。
实施例1
5-苯基-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物时使用苯甲醛)
白色固体,产率96%
1hnmr(600mhz,cdcl3)δ9.08(s,1h),7.42–7.37(m,3h),7.25–7.20(m,2h),7.17–7.10(m,5h),7.08–7.02(m,3h),6.64(t,j=7.2hz,1h),5.60(s,1h),4.52(dd,j=15.6,7.8hz,1h),4.28(dd,j=15.6,7.8hz,1h),4.15–4.08(m,2h),2.34(s,3h),1.26(t,j=6.6hz,3h).
13cnmr(150mhz,cdcl3)δ166.1,144.5,144.3,142.9,140.1,135.0,133.1,132.5,132.3,129.7,128.4,127.4,127.2,126.4,122.5,120.0,118.7,110.6,101.9,61.2,45.2,38.3,21.5,14.2.
实施例2
5-(4-氟苯基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用4-氟苯甲醛)
白色固体,产率95%
1hnmr(600mhz,cdcl3)δ9.10(s,1h),7.39(d,j=8.4hz,3h),7.24–7.19(m,2h),7.14(d,j=7.8hz,2h),7.06–7.02(m,3h),6.83(t,j=9.0hz,2h),6.67(t,j=9.0hz,1h),5.55(s,1h),4.54(dd,j=15.6,7.8hz,1h),4.24(dd,j=15.6,7.8hz,1h),4.14–4.08(m,2h),2.35(s,3h),1.26(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ166.10,144.32,139.78,138.60,135.03,133.25,132.49,132.34,129.68,128.77,128.72,127.36,122.59,120.06,118.56,115.20,115.06,110.69,102.05,61.23,45.27,37.75,21.51,14.17.
实施例3
5-(4-氯苯基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用4-氯苯甲醛)
白色固体,产率94%
1hnmr(600mhz,cdcl3)δ9.09(s,1h),7.43–7.34(m,3h),7.26–7.20(m,2h),7.12(dd,j=21.6,7.8hz,4h),7.09–7.04(m,1h),7.03–7.00(m,2h),6.66(t,j=7.2hz,1h),5.54(s,1h),4.54(dd,j=15.6,7.8hz,1h),4.22(dd,j=15.6,7.8hz,1h),4.15–4.07(m,2h),2.35(s,3h),1.26(t,j=6.6hz,3h).
13cnmr(150mhz,cdcl3)δ166.05,144.36,141.38,139.46,134.95,133.47,132.51,132.28,132.23,129.69,128.60,128.47,127.33,127.08,122.62,120.09,118.50,110.71,101.72,61.28,45.25,37.79,21.54,14.17.
实施例4
5-(4-溴苯基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用4-溴苯甲醛)
白色固体,产率93%
1hnmr(600mhz,cdcl3)δ9.10(s,1h),7.40–7.38(m,3h),7.25–7.24(m,2h),7.21(t,j=7.8hz,2h),7.14(t,j=8.4hz,2h),7.05(t,j=8.4hz,1h),6.94(d,j=8.4hz,2h),6.68(t,j=7.2hz,1h),5.52(s,1h),4.54(dd,j=15.6,7.8hz,1h),4.22(dd,j=15.6,7.8hz,1h),4.15–4.06(m,2h),2.35(s,3h),1.25(t,j=6.6hz,3h).
13cnmr(150mhz,cdcl3)δ166.04,144.37,141.92,139.39,134.94,133.50,132.52,132.28,131.42,129.69,128.99,127.33,127.06,122.63,120.35,120.10,118.48,110.72,101.61,61.29,45.24,37.84,21.54,14.16.
实施例5
5-(4-甲基苯基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用4-甲基苯甲醛)
白色固体,产率94%
1hnmr(600mhz,cdcl3)δ9.06(s,1h),7.40–7.38(m,3h),7.26–7.20(m,2h),7.14(d,j=8.4hz,2h),7.03(t,j=7.8hz,1h),6.96(s,4h),6.63(t,j=8.4hz,1h),5.56(s,1h),4.52(dd,j=15.6,7.8hz,1h),4.30(dd,j=15.6,7.8hz,1h),4.15–4.06(m,2h),2.34(s,3h),2.24(s,3h),1.25(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ166.14,144.21,140.14,139.89,136.02,135.00,132.97,132.49,132.28,129.64,129.06,127.36,127.33,127.08,122.43,119.90,118.74,110.57,102.12,61.10,45.26,37.96,21.52,20.91,14.18.
实施例6
5-(4-甲氧基苯基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用4-甲氧基苯甲醛)
白色固体,产率96%
1hnmr(600mhz,cdcl3)δ9.05(s,1h),7.40–7.38(m,3h),7.26–7.20(m,2h),7.14(d,j=8.4hz,2h),7.03(t,j=7.8hz,1h),6.99(d,j=8.4hz,2h),6.68(d,j=9.0hz,2h),6.63(t,j=7.8hz,1h),5.53(s,1h),4.53(dd,j=15.6,7.8hz,1h),4.29(dd,j=15.6,7.8hz,1h),4.14–4.06(m,2h),3.71(s,3h),2.34(s,3h),1.25(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ166.16,158.03,144.22,140.13,134.98,132.86,132.43,132.30,129.64,128.24,127.36,127.28,127.07,122.45,119.90,118.76,113.67,110.58,102.34,61.11,55.13,45.27,37.65,21.52,14.19.
实施例7
5-(4-硝基苯基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用4-硝基苯甲醛)
白色固体,产率91%
1hnmr(600mhz,cdcl3)δ9.17(s,1h),7.98(d,j=9.0hz,2h),7.42–7.40(m,3h),7.25–7.23(m,3h),7.19(d,j=8.4hz,1h),7.15(d,j=8.4hz,2h),7.07(t,j=7.8hz,1h),6.76(t,j=7.8hz,1h),5.66(s,1h),4.56(dd,j=15.6,7.8hz,1h),4.20–4.09(m,3h),2.36(s,3h),1.27(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ165.89,150.28,146.45,144.57,138.47,134.89,134.30,132.60,132.29,129.76,128.10,127.29,126.78,123.62,122.86,120.34,118.13,110.91,101.00,61.53,45.30,38.34,21.53,14.16.
实施例8
5-(3-甲基苯基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用3-甲基苯甲醛)
白色固体,产率95%
1hnmr(600mhz,cdcl3)δ9.09(s,1h),7.40–7.39(m,3h),7.24–7.20(m,2h),7.14(d,j=9.0hz,2h),7.05–7.02(m,2h),6.94(d,j=7.2hz,1h),6.90(s,1h),6.85(d,j=7.8hz,1h),6.63(t,j=7.8hz,1h),5.57(s,1h),4.53(dd,j=15.6,7.8hz,1h),4.32(dd,j=15.6,7.8hz,1h),4.15–4.08(m,2h),2.34(s,3h),2.21(s,3h),1.26(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ166.13,144.22,142.82,140.14,137.98,135.04,132.99,132.53,132.27,129.65,128.21,127.86,127.40,127.34,127.26,124.34,122.40,119.90,118.74,110.56,101.92,61.11,45.21,38.19,22.60,21.49,14.18.
实施例9
5-(3-甲氧基苯基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用3-甲氧基苯甲醛)
白色固体,产率97%
1hnmr(600mhz,cdcl3)δ9.07(s,1h),7.39–7.37(m,3h),7.26–7.19(m,2h),7.14(d,j=7.8hz,2h),7.08–7.02(m,2h),6.67–6.63(m,4h),5.57(s,1h),4.52(dd,j=15.6,7.8hz,1h),4.32(dd,j=15.6,7.8hz,1h),4.15–4.06(m,2h),3.67(s,3h),2.33(s,3h),1.25(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ166.08,159.54,144.52,144.22,139.85,135.04,133.22,132.53,132.25,129.64,129.29,127.34,122.44,119.95,119.71,118.67,113.74,111.10,110.59,101.79,61.14,55.03,45.19,38.21,29.03,21.51,14.18.
实施例10
5-(3-氯苯基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用3-氯苯甲醛)
白色固体,产率93%
1hnmr(600mhz,cdcl3)δ9.12(s,1h),7.40–7.38(m,3h),7.24–7.21(m,2h),7.14(d,j=8.4hz,2h),7.11–7.05(m,4h),6.94(d,j=7.8hz,1h),6.68(t,j=7.8hz,1h),5.56(s,1h),4.54(dd,j=15.6,7.8hz,1h),4.23(dd,j=15.6,7.8hz,1h),4.15–4.09(m,2h),2.34(s,3h),1.26(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ165.95,144.93,144.35,139.35,134.93,134.32,133.57,132.63,132.25,129.71,129.58,127.35,127.30,126.73,125.47,122.60,120.13,118.47,110.71,101.21,61.32,45.20,37.98,22.61,21.54,14.17.
实施例11
5-(3-溴苯基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用3-溴苯甲醛)
白色固体,产率92%
1hnmr(600mhz,cdcl3)δ9.12(s,1h),7.40–7.38(m,3h),7.25–7.21(m,4h),7.14(d,j=7.8hz,2h),7.07–7.05(m,1h),7.02–6.97(m,2h),6.68(t,j=7.8hz,1h),5.56(s,1h),4.55(dd,j=15.6,7.8hz,1h),4.23(dd,j=15.6,7.8hz,1h),4.15–4.09(m,2h),2.34(s,3h),1.26(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ165.93,145.22,144.34,139.30,134.94,133.59,132.64,132.25,130.22,129.89,129.71,129.67,127.30,127.09,125.94,122.67,122.61,120.13,118.46,110.72,101.16,61.32,45.20,37.97,21.53,14.16.
实施例12
5-(2-氯苯基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用2-氯苯甲醛)
白色固体,产率92%
1hnmr(600mhz,cdcl3)δ9.15(s,1h),7.42–7.39(m,3h),7.30(d,j=8.4hz,1h),7.26–7.20(m,2h),7.16(d,j=7.8hz,1h),7.08(t,j=7.8hz,1h),7.04(t,j=7.8hz,2h),6.95(t,j=7.8hz,1h),6.88(d,j=7.8hz,1h),6.44(t,j=7.2hz,1h),5.65(s,1h),4.52(dd,j=15.6,7.8hz,1h),4.40(dd,j=15.6,7.8hz,1h),4.12(q,j=14.4,7.2hz,2h),2.36(s,3h),2.05(s,3h),
13cnmr(150mhz,cdcl3)δ166.28,144.37,140.05,139.18,135.29,133.17,132.62,132.27,132.06,130.19,130.12,129.76,128.14,127.26,126.69,126.58,122.58,120.05,118.65,110.68,101.75,60.93,44.93,37.52,21.54,14.07.
实施例13
5-(3,4-二甲氧基苯基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用3,4-二甲氧苯甲醛)
白色固体,产率84%
1hnmr(600mhz,cdcl3)δ9.05(s,1h),7.40–7.38(m,3h),7.22(t,j=7.8hz,2h),7.14(d,j=8.4hz,2h),7.03(t,j=7.8hz,1h),6.75(s,1h),6.64(t,j=7.2hz,1h),6.60(d,j=8.4hz,1h),6.53–6.51(m,1h),5.52(s,1h),4.54(dd,j=15.6,7.8hz,1h),4.35(dd,j=15.6,7.8hz,1h),4.14–4.07(m,2h),3.77(s,3h),3.71(s,3h),2.34(s,3h),1.25(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ166.27,148.74,147.54,144.24,139.86,135.39,135.00,133.02,132.51,132.31,129.63,127.36,127.29,122.47,119.91,119.24,118.74,110.73,110.66,110.59,102.16,61.11,55.70,45.19,38.06,21.52,14.22.
实施例14
5-(2,4-二氯苯基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用2,4-二氯苯甲醛)
白色固体,产率94%
1hnmr(600mhz,cdcl3)δ9.15(s,1h),7.42–7.39(m,3h),7.31(s,1h),7.27–7.26(m,1h),7.25–7.24(m,1h),7.16(d,j=7.8hz,2h),7.06(t,j=7.2hz,1h),6.90(d,j=8.4hz,1h),6.81(d,j=8.4hz,1h),6.50(t,j=7.2hz,1h),5.61(s,1h),4.52(dd,j=15.6,7.8hz,1h),4.38(dd,j=15.6,7.8hz,1h),4.07–4.00(m,2h),2.37(s,3h),1.21(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ166.08,144.51,138.75,138.33,135.23,133.80,133.15,132.66,132.49,132.29,130.96,129.79,127.22,126.81,126.44,122.73,120.18,118.56,110.78,101.84,61.06,45.11,37.08,21.55,14.08.
实施例15
5-(萘-2-基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯
(合成底物使用2-萘甲醛)
白色固体,产率89%
1hnmr(600mhz,cdcl3)δ9.17(s,1h),7.74–7.73(m,1h),7.66(d,j=9.0hz,1h),7.61–7.60(m,1h),7.44–7.38(m,6h),7.29(d,j=9.0hz,1h),7.27–7.26(m,1h),7.28–7.21(m,1h),7.14(d,j=7.8hz,2h),7.02(t,j=7.2hz,1h),6.70(t,j=7.2hz,1h),5.77(s,1h),4.53(dd,j=15.6,7.8hz,1h),4.39(dd,j=15.6,7.8hz,1h),4.18–4.12(m,2h),2.34(s,3h),1.28(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ166.19,144.27,140.33,139.75,135.02,133.49,133.11,132.65,132.35,132.12,129.66,128.20,127.87,127.39,127.34,125.96,125.77,125.64,125.62,122.48,120.00,118.71,110.64,101.93,61.22,45.22,38.48,21.52,14.19.
实施例15产物的单晶数据(参见图1和下表):
表crystaldataandstructurerefinementforlq-180301.
实施例16
5-(吡啶-2-基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用2-吡啶甲醛)
白色固体,产率86%
1hnmr(600mhz,cdcl3)δ9.02(s,1h),dd(m,1h),7.51–7.48(m,1h),7.38(d,j=7.8hz,2h),7.33–7.29(m,2h),7.23(d,j=7.8hz,1h),7.19–7.16(m,1h),7.12(d,j=7.8hz,2h),7.04–7.01(m,2h),6.76(t,j=8.4hz,1h),5.62(s,1h),5.06(dd,j=15.6,7.2hz,1h),4.55(dd,j=15.6,7.8hz,1h),4.07(q,j=13.2,7.2hz,2h),2.33(s,3h),1.23(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ165.76,161.99,149.79,144.09,138.31,136.36,135.08,134.57,132.56,132.28,129.56,127.41,127.08,122.35,122.29,121.61,119.76,118.34,110.70,102.26,61.01,45.90,42.13,21.52,14.17.
实施例17
5-(噻吩-2-基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用2-噻吩甲醛)
白色固体,产率92%
1hnmr(600mhz,cdcl3)δ9.00(s,1h),7.39–7.34(m,4h),7.24–7.21(m,1h),7.13(d,j=9.0hz,2h),7.09–7.07(m,1h),7.05(dd,j=4.8,1.2hz,1h),6.78(q,j=5.4,3.0hz,1h),6.72–6.69(m,1h),6.66–6.65(m,1h),5.73(s,1h),4.60(dd,j=15.6,7.2hz,1h),4.46(dd,j=15.6,7.8hz,1h),4.12–4.10(m,2h),2.33(s,3h),1.27(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ165.43,146.67,144.32,138.97,134.86,133.89,132.17,132.06,129.66,127.37,126.99,126.39,124.67,124.02,122.59,120.03,118.63,110.66,102.59,61.23,45.30,34.78,21.52,14.20.
实施例18
5-(呋喃-2-基)-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物使用2-呋喃甲醛)
白色固体,产率91%
1hnmr(600mhz,cdcl3)δ8.96(s,1h),7.45(d,j=9.0hz,1h),7.37–7.35(m,3h),7.24–7.21(m,1h),7.19(dd,j=1.8,0.6hz,1h),7.13–7.09(m,3h),6.78(t,j=14.4hz,1h),6.15(dd,j=3.0,1.8hz,1h),5.91–5.90(m,1h),5.58(s,1h),4.62(dd,j=7.2,1.2hz,2h),4.10–4.06(m,2h),2.32(s,3h),1.24(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ165.21,154.16,144.25,141.83,136.38,134.93,134.78,132.20,132.12,129.62,127.37,126.94,122.51,119.91,118.58,110.62,109.98,106.38,100.61,61.13,45.50,33.64,21.50,14.16.
实施例19
8-甲基-5-苯基-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物时使用苯甲醛,6-甲基吲哚(其余此处未做说明的实施例均使用吲哚))
白色固体,产率94%
1hnmr(600mhz,cdcl3)δ8.92(s,1h),7.37(d,j=8.4hz,2h),7.19–7.06(m,9h),6.86(d,j=8.4hz,1h),6.63(t,j=7.2hz,1h),5.56(s,1h),4.51(dd,j=15.6,7.8hz,1h),4.26(dd,j=15.6,7.8hz,1h),4.13–4.07(m,2h),2.46(s,3h),2.34(s,3h),1.25(t,j=6.6hz,3h).
13cnmr(150mhz,cdcl3)δ166.15,144.17,142.94,139.93,134.99,133.13,132.68,132.43,131.89,129.62,128.36,127.37,127.23,126.38,125.14,121.59,118.46,110.62,101.98,61.13,45.31,38.39,21.74,21.53,14.19.
实施例20
7-溴-5-苯基-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物时使用苯甲醛,5-溴吲哚(其余此处未做说明的实施例均使用吲哚))
白色固体,产率89%
1hnmr(600mhz,cdcl3)δ9.15(s,1h),7.37–7.35(m,3h),7.30–7.28(m,1h),7.27–7.25(m,1h),7.17–7.14(m,5h),7.02(d,j=7.2hz,2h),6.64(t,j=7.8hz,1h),5.51(s,1h),4.50(dd,j=15.0,7.8hz,1h),4.23(dd,j=15.0,7.8hz,1h),4.15–4.09(m,2h),2.35(s,3h),1.26(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ165.97,144.49,142.36,140.16,134.83,133.57,133.07,130.79,129.75,129.11,128.49,127.29,127.09,126.61,125.38,121.11,113.28,112.12,101.40,61.28,45.02,37.95,21.55,14.20.
实施例21
1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4,5-二羧酸乙酯(合成底物时使乙醛酸乙酯)
白色固体,产率92%
1hnmr(600mhz,cdcl3)δ8.99(s,1h),7.65(d,j=7.8hz,1h),7.35(d,j=9.0hz,3h),7.25–7.23(m,1h),7.17–7.15(m,1h),7.11(d,j=7.8hz,2h),6.89(t,j=7.8hz,1h),5.16(s,1h),4.73(dd,j=14.4,7.2hz,1h),4.64(dd,j=15.6,7.8hz,1h),4.10–4.02(m,4h),2.31(s,3h),1.24(t,j=7.2hz,3h),1.16(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ170.91,165.07,144.29,137.03,134.95,133.93,132.61,131.96,129.66,127.32,126.89,122.59,120.04,118.99,110.63,98.39,61.68,61.22,45.91,40.74,21.50,14.14,14.01.
实施例22
5-戊基-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物时使用己醛)
白色固体,产率91%
1hnmr(600mhz,cdcl3)δ8.81(s,1h),7.49(d,j=8.4hz,1h),7.36–7.35(m,3h),7.24–7.21(m,1h),7.15–7.10(m,3h),6.70(t,j=7.2hz,1h),4.67(t,j=6.6hz,2h),4.21(dd,j=8.4,5.4hz,1h),4.09–4.05(m,2h),2.31(s,3h),1.58–1.46(m,3h),1.25(t,j=7.2hz,3h),1.22–1.07(m,7h).
13cnmr(150mhz,cdcl3)δ166.39,144.08,138.97,135.19,133.31,132.36,130.69,129.54,127.32,126.91,122.35,119.58,118.48,110.71,106.64,60.89,46.20,37.95,34.03,31.77,27.49,22.50,21.47,14.17,14.03.
实施例23
5-异丁基-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物时使用异戊醛)
白色固体,产率93%
1hnmr(600mhz,cdcl3)δ8.82(s,1h),7.47(d,j=7.8hz,1h),7.36(t,j=8.4hz,3h),7.24–7.21(m,1h),7.15–7.11(m,3h),6.69(t,j=7.2hz,1h),4.62–4.53(m,2h),4.31(dd,j=9.6,4.8hz,1h),4.06(q,j=13.8,7.2hz,2h),2.31(s,3h),1.53–1.41(m,2h),1.38–1.28(m,2h),1.25(t,j=7.2hz,3h),0.95(d,j=5.4hz,2h),0.91–0.86(m,1h),0.84(d,j=6.6hz,4h).
13cnmr(150mhz,cdcl3)δ166.41,144.10,139.50,135.22,133.45,132.26,130.84,129.57,127.31,126.68,122.35,119.62,118.17,110.74,106.72,60.92,47.99,46.08,31.63,26.12,23.63,21.96,21.47,14.14.
实施例24
5-环己基-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物时使用环己醛)
白色固体,产率82%
1hnmr(600mhz,cdcl3)δ9.00(s,1h),7.52(d,j=8.4hz,1h),7.37–7.34(m,3h),7.22–7.19(m,1h),7.13–7.11(m,3h),6.65(t,j=7.2hz,1h),4.64–4.55(m,2h),4.09–4.04(m,3h),2.31(s,3h),1.53–1.42(m,6h),1.23(t,j=7.2hz,3h),1.03–0.89(m,5h).
13cnmr(150mhz,cdcl3)δ166.78,144.21,139.20,135.07,132.48,131.91,130.78,129.66,127.91,127.23,122.04,119.47,119.21,110.55,105.40,60.92,46.28,45.95,39.32,32.17,31.05,26.37,26.11,21.48,14.15.
实施例25
5-环戊基-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸乙酯(合成底物时使用环戊醛)
白色固体,产率87%
1hnmr(600mhz,cdcl3)δ8.93(s,1h),7.54(d,j=8.4hz,1h),7.40–7.38(m,2h),7.35–7.33(m,1h),7.22–7.19(m,1h),7.13–7.10(m,3h),6.64(t,j=6.6hz,1h),4.74(dd,j=17.4,6.6hz,1h),4.54(dd,j=16.8,6.0hz,1h),4.12–4.06(m,3h),2.32(s,3h),1.53–1.37(m,3h),1.24(t,j=7.2hz,3h),1.22–1.11(m,6h).
13cnmr(150mhz,cdcl3)δ166.91,144.14,138.50,135.40,132.79,132.10,130.05,129.62,127.19,122.14,119.45,118.99,110.61,107.93,60.96,49.42,46.39,37.55,32.43,30.70,24.13,23.80,21.48,14.17.
实施例26
5-环戊基-1-对甲苯磺酰基-1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-羧酸2-氧代-2-苯乙基酯(合成底物时使用苯甲醛,硫盐使用14)
白色固体,产率92%
1hnmr(600mhz,cdcl3)δ9.13(s,1h),7.93–7.92(m,2h),7.65–7.63(m,1h),7.53–7.50(m,2h),7.44(d,j=7.8hz,2h),7.39(d,j=7.8hz,1h),7.25–7.20(m,1h),7.18–7.16(m,3h),7.15–7.11(m,4h),7.05–7.02(m,1h),6.85(t,j=7.2hz,1h),5.62(s,1h),5.31(q,j=29.4,16.2hz,2h),4.56(dd,j=15.6,7.8hz,1h),4.30(dd,j=15.0,7.2hz,1h),2.34(s,3h).
13cnmr(150mhz,cdcl3)δ192.30,166.70,145.53,143.64,140.59,135.83,135.55,135.16,134.98,133.50,133.25,130.90,129.96,129.41,128.71,128.42,128.30,128.28,127.45,123.44,121.01,119.62,111.60,102.77,67.51,46.16,39.39,22.61.
实施例27
a还原:
以化合物1(0.05mmol)为原料,加入反应试管,加入1ml二氯甲烷做溶剂以及dibalh(0.055mmol)。在-78℃下反应1h,使用薄层色谱监测反应,待反应完成后,将反应液倒入水中,使用二氯甲烷萃取,浓缩有机层,得到的粗产品使用柱层析纯化,使用石油醚:乙酸乙酯=4:1分离得到产物。
5-苯基-1-甲苯磺酰基1,2,5,10-四氢吖庚因并[2,3-b]吲哚-4-基)甲醇
白色固体,产率95%
1hnmr(600mhz,cdcl3)δ9.03(s,1h),7.44–7.42(m,2h),7.39–7.37(m,1h),7.21–7.12(m,9h),7.02–6.99(m,1h),5.51(t,j=7.2hz,1h),4.73(s,1h),4.45(dd,j=15.6,7.8hz,1h),4.28(dd,j=15.6,7.2hz,1h),3.84–3.78(m,2h),2.40(s,3h).
13cnmr(150mhz,cdcl3)δ148.6,144.3,143.1,136.0,132.6,132.4,129.4,128.5,127.8,127.4,127.3,126.6,122.4,119.8,119.4,118.7,110.6,103.2,66.8,46.2,41.5,21.5.
实施例28
b还原:
以实施例27的产物(0.05mmol)为原料,加入反应试管,加入1ml乙醇做溶剂以及pd(oh)2(0.01mmol)和大量氢气。在室温下反应12h,使用薄层色谱监测反应,待反应完成后,浓缩溶剂,得到的粗产品使用柱层析纯化,使用石油醚:乙酸乙酯=4:1分离得到产物。
(5-苯基-1-甲苯磺酰基1,2,3,4,5,10-四氢吖庚因并[2,3-b]吲哚-4-基)甲醇
白色固体,产率97%
1hnmr(600mhz,cdcl3)δ8.93(s,1h),7.67(d,j=8.4hz,2h),7.31–7.30(m,1h),7.27–7.25(m,3h),7.14–7.11(m,1h),7.07–7.01(m,2h),6.99–6.96(m,2h),6.92–6.89(m,1h),6.60(d,j=7.2hz,2h),4.28–4.23(m,1h),4.07(d,j=9.0hz,1h),3.87–3.83(m,1h),3.48(dd,j=10.8,4.8hz,1h),3.32(dd,j=10.8,6.6hz,1h),2.45(s,3h),2.06–2.01(m,1h),1.95–1.90(m,1h),1.80–1.73(m,1h).
13cnmr(150mhz,cdcl3)δ144.2,143.3,136.1,133.3,130.0,128.1,128.0,127.5,127.4,126.1,122.2,119.6,119.0,110.6,107.6,64.7,49.7,44.0,43.1,27.9,21.7.
实施例29
c氧化
以实施例1的产物(0.05mmol)为原料,加入反应试管,加入1ml四氢呋喃做溶剂以及tbaf(0.25mmol)。在60℃下回流4h,使用薄层色谱监测反应,待反应完成后,将反应液倒入水中,使用二氯甲烷萃取,浓缩有机层,得到的粗产品使用柱层析纯化,使用石油醚:乙酸乙酯=6:1分离得到产物。
5-苯基-5,10-二氢氮杂吖庚因并[2,3-b]吲哚-4-羧酸乙酯
红色固体,产率98%
1hnmr(600mhz,cdcl3)δ9.31(s,1h),7.98(d,j=4.2hz,1h),7.77(d,j=7.8hz,1h),7.39(d,j=8.4hz,1h),7.31–7.28(m,2h),7.22(dd,j=4.8,1.2hz,1h),7.18–7.15(m,3h),7.13–7.10(m,1h),7.08–7.06(m,2h),6.08(s,1h),4.38–4.32(m,2h),1.38(t,j=7.2hz,3h).
13cnmr(150mhz,cdcl3)δ166.5,151.7,142.1,141.7,138.3,137.3,128.3,127.5,126.8,126.7,126.0,124.4,120.3,119.5,111.1,106.2,62.0,38.0,14.2.
实施例29产物的单晶数据(见图2和下表):
表crystaldataandstructurerefinementforlq-qyh-2.
以下通过试验例来具体说明本发明的有益效果。
试验例1、抗肿瘤研究
1、实验肿瘤细胞株
人乳腺癌mb468细胞株、人乳腺癌skbr3细胞株、人乳腺癌mb231细胞株、小鼠黑色素瘤a375细胞株均由四川大学生物治疗国家重点实验室提供,以上肿瘤细胞均冻存于四川大学生物治疗国家重点实验室。
2、实验方法
2.1细胞的准备及处理
4种肿瘤细胞均培养于含10%灭活新生小牛血清的rpmi-1640培养液,37℃、5%co2培养箱中生长至80%细胞融合,用0.1%胰酶溶液消化,制成单细胞悬液,调整细胞浓度为5×104个/ml,均匀接种于96孔微量培养板中,每组3个复孔,100μl/孔,置37℃饱和湿度、5%co2孵箱内培养24h后,正常对照组加入含等量的培养液;加入浓度梯度的受试药物(100、50、25、12.5、6.25μg/ml),每个浓度设3个复孔,实验平行2次。待药物与细胞作用24h后,每孔加入10μlmtt溶液(5mg/ml),继续培养4h后每孔加入100μldmso,振荡混匀,使结晶物充分溶解,在酶标仪490nm波长处测其吸光度值(a值),各个浓度组取其平均值。
2.2肿瘤细胞增殖抑制率的测定
按下列公式计算细胞增殖抑制率:细胞增殖抑制率(%)=(1-试验组a值/对照组a值)×100%。所有实验数据采用spss13.0进行统计分析。实验结果采用probit求得ic50值。
3、实验结果
表1本发明化合物对受试细胞生长的抑制情况
实验结果表明,本发明化合物具有优异的抗肿瘤效果。(以上数值大于50才具有抗肿瘤效果)
综上所述,本发明化合物的制备方法简便、反应温和、收率高,并且具有优良的抗肿瘤活性,具有广阔的市场应用前景。
- 还没有人留言评论。精彩留言会获得点赞!