硒/硫化磺酰芳胺类化合物及其硒/硫化方法与流程
本发明属于化学合成领域,特别涉及导向基团辅助的过渡金属催化c(sp2)—h硒(硫)化反应。
背景技术:
近年来,有机硒(硫)类化合物被广泛地应用于有机合成,功能性材料,荧光探针等领域。另外,该类化合物表现出抗肿瘤,抗氧化,抗炎,抗菌,抗病毒等生物活性,因此在药物开发领域也具有重要意义。
利用传统合成方法,有机硒(硫)类化合物主要通过芳基卤化物、芳胺或者芳基硼酸化物与硒(硫)醚(醇)反生偶联反应得到。该方法存在底物局限,步骤繁琐,底物需要预官能团化等不足。
过渡金属催化c(sp2)—h直接硒(硫)化已经成为合成有机硒(硫)类化合物的有效策略。ackermann,nishihara,kambe,jana,baidya,zhang等课题组已经对导向基团辅助的过渡金属催化c(sp2)—h直接硒(硫)化反应做出了相关报道,但是他们构建的方法多以8-氨基喹啉,吡啶,嘧啶,肟,氮氧化合物等强配位作用基团作为导向基团。以磺胺为导向基团辅助的硒(硫)化反应未见文献报道。
通过弱配位导向基团辅助的硒(硫)化反应报道较少。ackermannn报道了通过酰胺氧弱配位辅助的铜催化c—h硒(硫)化反应。随后,baidya首次构建了钌催化的芳基羧酸衍生物邻位c—h硒(硫)化反应方法。这些方法能以高化学和位点选择性合成有机硒(硫)化合物,但是仍存在反应体系复杂,反应时间长,后处理繁琐等不足。开发操作简捷,底物适用范围广,官能团耐受性好,化学和位点选择性高的有机硒(硫)类化合物合成方法具有重要意义。
技术实现要素:
本发明提的目的在于提供硒/硫化磺酰芳胺类化合物及其硒/硫化方法。
本发明文字中,“/”表示“或”的关系。
本发明以磺酰胺为导向基团,提供钯催化的磺酰芳胺类化合物邻位c(sp2)—h硒/硫化反应方法即得到的硒/硫化磺酰芳胺类化合物,通过弱配位作用构建过渡金属钯催化邻位c(sp2)—h选择性硒/硫化反应方法,合成一系列硒/硫化磺酰芳胺类衍生物。所述硒/硫化磺酰芳胺类化合物具有以下结构通式:
式中,x为se(硒)或s(硫);
r1为h,-ch3,-cl,-f,-br,-cf3,-et,-och3,1,3二氧五环,1,4二氧六环,苯环,溴苯中的一种,r1为对位和/或间位取代);
当为1,3二氧五环或1,4二氧六环时,连接方式为与苯环并环连接;当为苯环或溴苯时候,连接方式为呈萘环连接。此时结构通式依次为:
r2为-ch3、-et、环丙基、-ph(苯基),-bn(苄基),
r3为-ph(苯基),
本发明提供的上述化合物的制备方法,包括以下步骤:
将磺酰芳胺类底物、二芳基二硒/硫醚混合按照摩尔比1:2混合,加入钯催化剂和氧化剂、溶剂,在氩气保护下于100~125℃反应10~15h,反应结束后进行纯化,得到硒/硫化磺酰芳胺类化合物。
进一步地,所述钯催化剂为pd(tfa)2或pd(oac)2;优选为pd(tfa)2。
进一步地,所述氧化剂为ag2o、ag2co3、agoac、agcf3co2、agno3、cu(oac)2、cu(cf3co2)2、m-cpba、k2o8s2、tbuooh、phi(oac)2、cu(oac)2,优选为cu(oac)2。
进一步地,所述纯化方法为将反应所得反应液加水和乙酸乙酯萃取至少三次,然后合并有机层,无水na2so4干燥有机层,过滤,浓缩,硅胶柱层析,得到。优选地,硅胶柱层析的流动相选用石油醚/乙酸乙酯体积比为(5-30):1。
进一步地,所述溶剂优选为甲苯。
进一步地,所述催化剂的加入量为磺酰芳胺类底物的摩尔量的8-12%的摩尔量,优选为10%的摩尔量;所述氧化剂的加入量为磺酰芳胺类底物的1.5-2.5倍摩尔量,优选为2倍摩尔量。
进一步地,所述反应温度优选为110-125℃,进一步优选为125℃。
进一步地,所述溶剂的用量根据溶解反应物料和提供反应环境所需来确定,优选为每0.25mmol/3ml。
进一步地,所述磺酰芳胺类底物包括以下结构通式中的化合物中的一种:
式中,r1为h,-ch3,-cl,-f,-br,-cf3,-et,-och3中的一种;r1为对位和/或间位取代;
r2为-ch3、-et、环丙基、-ph(苯基),-bn(苄基),
所述二芳基二硒/硫醚的为以下结构通式的化合物:
r3xxr3
式中,x为se(硒)或s(硫);
r3为-ph(苯基),
本发明所述方法中,反应方程式如下:
式中,x为se(硒)或s(硫);
r1为h,-ch3,-cl,-f,-br,-cf3,-et,-och3,1,3二氧五环,1,4二氧六环,苯环,溴苯中的一种,r1为对位和/或间位取代;当为1,3二氧五环或1,4二氧六环时,连接方式为与苯环并环连接;当为苯环或溴苯时候,连接方式为呈萘环连接。即所述化合物1可以是取代的苯环或萘环。
r2为-ch3、-et、环丙基、-ph(苯基),-bn(苄基),
r3为-ph(苯基),
本发明提供的上述硒/硫化磺酰芳胺类化合物在制备抗菌药物中的应用。
磺胺类化合物是一类常用的抗菌药物,有机硒(硫)类化合物也具有抗炎作用。本发明利用过渡金属钯催化碳氢官能化的方法,对磺胺类化合物进行硒醚或者硫醚修饰,可得到抗菌活性更强,耐药性低的活性分子,可作为药物活性组分应用于制备抗菌药物中。
与现有技术相比,本发明具有以下有益效果:
1.本发明提供了一种操作简单,制备条件易得的磺胺药物的后阶段修饰方法。磺酰芳胺类底物结构简单易制备,衍生范围广,丰富了此类化合物的结构多样性,解决了传统反应条件苛刻,操作复杂,底物局限,步骤繁琐,底物的官能团不耐受等不足。
2.本发明提供的钯催化的磺酰芳胺类化合物邻位c(sp2)—h硒/硫化反应方法,具有优异的化学和位置选择性,在广泛的底物上提供了新的各种硒/硫化磺酰芳胺类衍生物。
3.本发明方法可应用于磺胺类药物的结构修饰,通过硒醚或者硫醚修饰可增强磺胺类的抗菌作用,得到抗菌活性更强,耐药性低的活性分子为新药开发奠定基础。
附图说明
图1-图19分为对图中对应化学结构式的化合物的氢谱图和碳谱图。
具体实施方式
下面结合具体实施例,进一步阐述本发明。以下所述仅为本发明的实施例,并非因此限制本发明的专利范围,凡是利用本发明说明书内容所作的等效结构或等效流程变换,或直接或间接运用在其他相关的技术领域,均同理包括在本发明的专利保护范围内。
实施例1
将0.25mmol磺酰芳胺类底物1和2倍摩尔量的二芳基二硒醚2混合,加入磺酰芳胺类底物摩尔量的10%的摩尔量的催化剂pd(tfa)2和2倍磺酰芳胺类底物摩尔量的氧化剂cu(oac)2到反应管中,在氩气保护下加入干燥甲苯3ml,125℃反应12h得混合液体,反应结束后添加水和乙酸乙酯萃取三次,合并有机层,无水na2so4干燥有机层,过滤,浓缩,硅胶柱层析(流动相:石油醚/乙酸乙酯=30/1—5/1)分离纯化得到硒化磺酰芳胺类化合物3。
r1为h,-ch3,-cl,-f,-br,-cf3,-et,-och3,1,3二氧五环,1,4二氧六环,苯环,溴苯中的一种,r1为对位和/或间位取代;
当为1,3二氧五环或1,4二氧六环时,连接方式为与苯环并环连接;当为苯环或溴苯时候,连接方式为呈萘环连接。此时结构通式依次为:
r2为-ch3、-et、环丙基、-ph(苯基),-bn(苄基),
r3为-ph(苯基),
所用反应物均为现有化合物,可于市场购买或根据现有技术合成。
按照以上方法,根据所需要合成的硒化磺酰芳胺类化合物的具体结构式,选择相应的磺酰芳胺类底物进行反应,如制备化合物3aa,结构式如下:
化合物3aa的制备:在反应管中加入n-苯基甲磺酰胺(1a)(42.7mg,0.25mmol),1,2-二苯基二硒醚(2a)(156.0mg,0.5mmol),pd(tfa)2(8.3mg,0.025mmol),cu(oac)2(91mg,0.5mmol),在氩气保护下加入干燥甲苯(3ml),125℃反应12h得混合液体,反应结束后添加水和乙酸乙酯萃取三次,合并有机层,无水na2so4干燥有机层,过滤,浓缩,硅胶柱层析(石油醚/乙酸乙酯=30/1—5/1)分离纯化得到3aa(66mg,81%)。
同理,选择相应的反应底物可得到以下结构式的硒化磺酰芳胺类化合物,以及对应收率如下:
实施例2
加入磺酰芳胺类底物摩尔量的10%的摩尔量的催化剂pd(tfa)2和2倍磺酰芳胺类底物摩尔量的氧化剂cu(oac)2到反应管中,在氩气保护下加入干燥甲苯3ml,125℃反应12h得混合液体,反应结束后添加水和乙酸乙酯萃取三次,合并有机层,无水na2so4干燥有机层,过滤,浓缩,硅胶柱层析(流动相:石油醚/乙酸乙酯=30/1—5/1)分离纯化得到硫化磺酰芳胺类化合物5。
r1为-ch3,-cl,-cf3,-et,-och3,1,3二氧五环;
r2为-ch3;
r3为-ph(苯基),
所用反应物均为现有化合物,可于市场购买或根据现有技术合成。
按照以上方法,根据所需要合成的硒化磺酰芳胺类化合物的具体结构式,选择相应的磺酰芳胺类底物进行反应,如制备化合物5a,结构式如下:
化合物5a的制备:在反应管中加入n-(对甲基苯基)甲磺酰胺(1b)(46.2mg,0.25mmol),1,2-二对甲苯基二硫醚(4a)(123.0mg,0.5mmol),pd(tfa)2(8.3mg,0.025mmol),cu(oac)2(91mg,0.5mmol),在氩气保护下加入干燥甲苯(3ml),125℃反应12h得混合液体,反应结束后添加水和乙酸乙酯萃取三次,合并有机层,无水na2so4干燥有机层,过滤,浓缩,硅胶柱层析(石油醚/乙酸乙酯=30/1—5/1)分离纯化得到5a(72mg,94%)。
同理,选择相应的反应底物可得到以下结构式的硫化磺酰芳胺类化合物,以及对应收率如下:
产品表征
对实施例1和实施例2制备的化合物产品进行表征,表征数据如下:
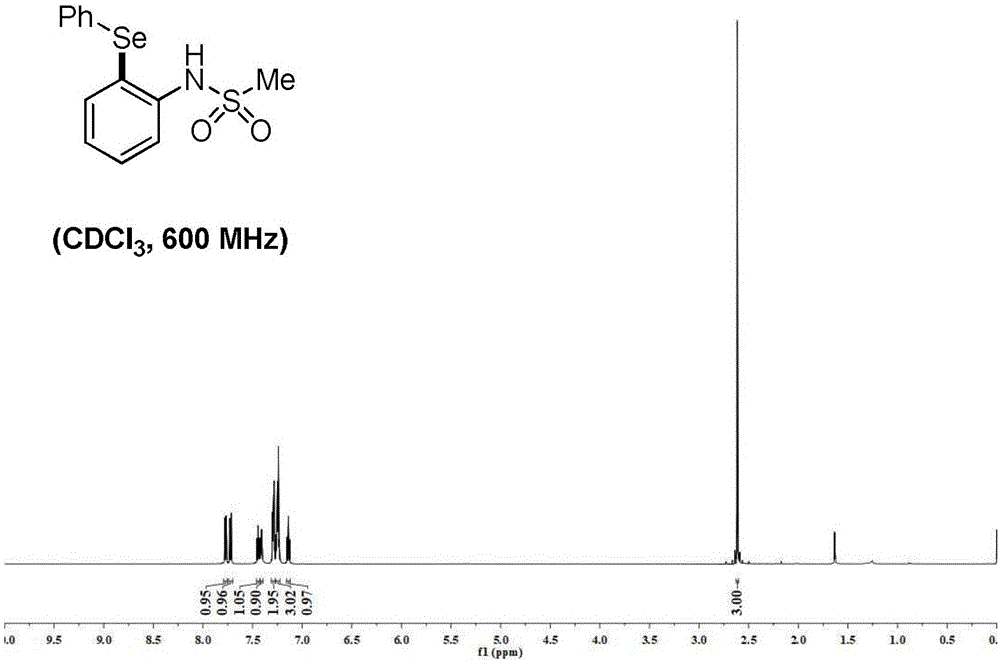
n-(2-(phenylselanyl)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.77(dd,j=7.6,1.4hz,1h),7.72(dd,j=8.2,1.1hz,1h),7.46–7.43(m,1h),7.41(s,1h),7.31–7.27(m,2h),7.27–7.22(m,3h),7.15–7.13(m,1h),2.61(s,3h).13cnmr(150mhz)δ=139.1,138.5,131.5,131.0,130.5,129.9,127.6,125.5,120.5,119.6,39.1.hr-ms(esi)m/zcalcdfor:c13h12no2s80se-[m-h-]325.9769,found325.9759.
n-(2-(phenylselanyl)phenyl)ethanesulfonamide:1hnmr(600mhz)δ=7.75–7.72(m,2h),7.45–7.39(m,2h),7.29–7.22(m,5h),7.12–7.09(m,1h),2.92(q,j=7.4hz,2h),1.17(t,j=7.4hz,3h).13cnmr(150mhz)δ=139.4,138.6,131.4,130.6,130.4,129.8,127.5,125.0,119.3,118.7,46.3,8.1.hr-ms(esi)m/zcalcdfor:c14h14no2s80se-[m-h-]339.9915,found339.9916.
n-(2-(phenylselanyl)phenyl)propane-2-sulfonamide:1hnmr(600mhz)δ=7.76(dd,j=8.3,1.1hz,1h),7.72(dd,j=7.8,1.6hz,1h),7.44–7.37(m,2h),7.25–7.21(m,5h),7.08–7.06(m,1h),3.21(hept,j=6.9hz,1h),1.25(d,j=6.9hz,6h).13cnmr(150mhz)δ=139.8,138.7,131.4,130.4,130.3,129.8,127.4,124.6,118.5,118.1,53.2,16.5.hr-ms(esi)m/zcalcdfor:c15h16no2s80se-[m-h-]354.0081,found354.0072.
n-(2-(phenylselanyl)phenyl)cyclopropanesulfonamide:1hnmr(600mhz)δ=7.82–7.66(m,2h),7.47–7.34(m,2h),7.32–7.17(m,5h),7.17–7.07(m,1h),2.17–2.09(m,1h),1.10–1.00(m,2h),0.79–0.67(m,2h).13cnmr(150mhz)δ=139.3,138.3,131.2,130.6,129.7,127.4,125.4,120.6,120.5,30.1,5.7.hr-ms(esi)m/zcalcdfor:c15h14fno2s80se-[m-h-]351.9915,found351.9916.
1-phenyl-n-(2-(phenylselanyl)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.72(dd,j=7.7,1.3hz,1h),7.69(d,j=8.2hz,1h),7.46(s,1h),7.43–7.38(m,1h),7.33(t,j=7.3hz,1h),7.28(t,j=7.5hz,2h),7.23–7.18(m,5h),7.12(d,j=7.4hz,2h),7.09(t,j=7.6hz,1h),4.14(s,2h).13cnmr(150mhz)δ=139.5,138.7,131.5,130.8,130.6,130.5,129.7,129.1,129.0,128.1,127.4,124.9,119.4,118.1,57.8.hr-ms(esi)m/zcalcdfor:c19h16no2s80se-[m-h-]402.0084,found402.0072.
n-(2-(phenylselanyl)phenyl)thiophene-2-sulfonamide:1hnmr(600mhz)δ=7.82–7.77(m,1h),7.74(s,1h),7.61(dd,j=7.6,1.3hz,1h),7.48–7.46(m,1h),7.43–7.39(m,1h),7.36(dd,j=3.6,1.1hz,1h),7.21–7.14(m,3h),7.10–7.06(m,3h),6.92(dd,j=4.9,3.9hz,1h).13cnmr(150mhz)δ=139.2,138.6,138.3,132.9,132.7,131.1,130.6,130.3,129.7,127.4,127.2,125.8,120.9,120.5.hr-ms(esi)m/zcalcdfor:c16h12no2s280se-[m-h-]393.9488,found393.9480.
4-methoxy-n-(2-(phenylselanyl)phenyl)benzenesulfonamide:1hnmr(600mhz)δ=7.72(dd,j=8.2,0.9hz,1h),7.67(s,1h),7.58(dd,j=7.6,1.3hz,1h),7.57–7.54(m,2h),7.38–7.34(m,1h),7.22–7.18(m,1h),7.17–7.14(m,2h),7.06–7.00(m,3h),6.78–6.74(m,2h),3.81(s,3h).13cnmr(150mhz)δ=163.2,139.1,138.4,131.1,130.8,130.3,130.1,129.6,129.5,127.0,125.2,120.0,119.7,114.2,55.6.hr-ms(esi)m/zcalcdfor:c19h16no3s80se-[m-h-]418.0034,found418.0022.
4-methyl-n-(2-(phenylselanyl)phenyl)benzenesulfonamide:1hnmr(600mhz)δ=7.73–7.68(m,2h),7.57(dd,j=7.7,1.4hz,1h),7.53–7.50(m,2h),7.37–7.33(m,1h),7.20(dd,j=8.3,6.3hz,1h),7.16–7.12(m,2h),7.10(d,j=8.1hz,2h),7.06–7.04(m,2h),7.03–7.00(m,1h),2.34(s,3h).13cnmr(150mhz)δ=144.0,139.0,138.4,135.8,131.1,130.7,130.2,129.7,129.6,127.4,127.0,125.2,120.0,119.7,21.7.hr-ms(esi)m/zcalcdfor:c19h16no2s80se-[m-h-]402.0083,found402.0072.
n-(4-methyl-2-(phenylselanyl)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.63–7.58(m,2h),7.30–7.28(m,2h),7.27–7.21(m,5h),2.59(s,3h),2.34(s,3h).13cnmr(150mhz)δ=138.7,136.5,135.6,132.1,130.9,130.6,129.8,127.5,120.6,120.1,38.9,20.7.hr-ms(esi)m/zcalcdfor:c14h14no2s80se-[m-h-]339.9924,found339.9916.
n-(4-ethyl-2-(phenylselanyl)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.63(d,j=8.3hz,1h),7.60(d,j=2.0hz,1h),7.30–7.20(m,7h),2.64(q,j=7.6hz,2h),2.60(s,3h),1.24(t,j=7.6hz,3h).13cnmr(150mhz)δ=141.9,137.6,136.7,130.9,130.8,130.6,129.8,127.5,120.7,120.2,39.0,28.1,15.5.hr-ms(esi)m/zcalcdfor:c15h16no2s80se-[m-h-]354.0080,found354.0072.
n-(4-methoxy-2-(phenylselanyl)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.62(d,j=8.9hz,1h),7.37–7.33(m,2h),7.30–7.27(m,3h),7.21(d,j=3.1hz,1h),6.95(dd,j=8.9,3.0hz,1h),6.91(s,1h),3.79(s,3h),2.63(s,3h).13cnmr(150mhz)δ=157.4,134.5,131.6,131.3,129.9,127.9,124.0,123.6,122.2,116.2,55.8,39.0.hr-ms(esi)m/zcalcdfor:c14h14no3s80se-[m-h-]355.9873,found355.9865.
n-(2-(phenylselanyl)-4-(trifluoromethyl)phenyl)methanesulfonamide:1hnmr(600mhz)δ=8.05(d,j=1.6hz,1h),7.82(d,j=8.5hz,1h),7.68(dd,j=8.5,1.8hz,1h),7.61(s,1h),7.34–7.27(m,5h),2.65(s,3h).13cnmr(150mhz)δ=142.2,135.2(q,j=3.1hz),131.4,130.1,129.4,128.5(q,j=2.9hz),128.2,126.9(q,j=33.4hz),123.4(q,j=272.6hz),120.4,118.2,39.6.hr-ms(esi)m/zcalcdfor:c14h11f3no2s80se-[m-h-]393.9644,found393.9633.
n-(4-chloro-2-(phenylselanyl)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.72(d,j=2.4hz,1h),7.65(d,j=8.8hz,1h),7.39(dd,j=8.8,2.5hz,1h),7.37–7.32(m,2h),7.31–7.26(m,4h),2.61(s,3h).13cnmr(150mhz)δ=137.4,137.1,131.7,131.2,130.4,130.1,129.5,128.2,122.6,121.0,39.3.hr-ms(esi)m/zcalcdfor:c13h11clno2s80se-[m-h-]359.9378,found359.9370.
n-(5-fluoro-2-(phenylselanyl)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.81–7.76(m,1h),7.53(s,1h),7.51–7.49(m,1h),7.26–7.23(m,5h),6.88–6.84(m,1h),2.62(s,3h).13cnmr(150mhz)δ=164.6(d,j=250.6hz),141.0(d,j=11.5hz),140.2(d,j=9.1hz),130.6,130.5,129.9,127.7,114.4,112.2(d,j=21.6hz),106.6(d,j=27.5hz),39.3.hr-ms(esi)m/zcalcdfor:c13h11fno2s80se-[m-h-]343.9672,found343.9665.
n-(5-chloro-2-(phenylselanyl)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.74(d,j=2.1hz,1h),7.71(d,j=8.2hz,1h),7.43(s,1h),7.30–7.27(m,2h),7.27–7.25(m,3h),7.12(dd,j=8.3,2.1hz,1h),2.63(s,3h).13cnmr(150mhz)δ=140.2,139.2,137.6,131.0,130.0,127.9,125.4,119.2,118.3,39.4.hr-ms(esi)m/zcalcdfor:c13h11clno2s80se-[m-h-]369.9378,found369.9370.
n-(4,5-dimethoxy-2-(phenylselanyl)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.35(s,1h),7.24–7.21(m,6h),7.14(s,1h),3.93(s,3h),3.89(s,3h),2.56(s,3h).13cnmr(150mhz)δ=151.4,146.7,133.3,131.4,130.0,129.8,127.3,120.3,110.6,105.1,56.4,56.3,38.8.hr-ms(esi)m/zcalcdfor:c15h16no4s80se-[m-h-]385.9983,found385.9971.
n-(6-(phenylselanyl)benzo[d][1,3]dioxol-5-yl)methanesulfonamide:1hnmr(600mhz)δ=7.31(s,1h),7.29–7.27(m,2h),7.26–7.21(m,4h),7.20(s,1h),6.04(s,2h),2.59(s,3h).13cnmr(150mhz)δ=150.3,145.4,134.0,131.1,130.4,129.8,127.5,116.9,112.0,102.9,102.2,39.0.hr-ms(esi)m/zcalcdfor:c14h12no4s80se-[m-h-]369.9663,found369.9658.
n-(7-(phenylselanyl)-2,3-dihydrobenzo[b][1,4]dioxin-6-yl)methanesulfonamide:1hnmr(600mhz)δ=7.30(s,1h),7.28(s,1h),7.28–7.20(m,6h),4.31–4.29(m,2h),4.28–4.24(m,2h),2.58(s,3h).13cnmr(150mhz)δ=146.0,141.2,133.0,131.0,130.5,129.8,127.4,126.5,112.0,110.0,64.6,64.3,38.8.hr-ms(esi)m/zcalcdfor:c15h14no4s80se-[m-h-]383.9824,found383.9814.
n-(1-(phenylselanyl)naphthalen-2-yl)methanesulfonamide:1hnmr(600mhz)δ=8.51(d,j=8.5hz,1h),8.08(s,1h),8.00–7.90(m,2h),7.85(d,j=8.1hz,1h),7.59–7.55(m,1h),7.48(t,j=7.6hz,1h),7.15–7.12(m,5h),2.65(s,3h).13cnmr(150mhz)δ=139.1,136.1,132.5,131.4,130.9,129.8,129.7,128.7,128.6,128.5,127.2,125.8,118.3,117.0,39.7.hr-ms(esi)m/zcalcdfor:c17h14no2s80se-[m-h-]375.9924,found375.9916.
n-(6-bromo-1-(phenylselanyl)naphthalen-2-yl)methanesulfonamide:1hnmr(600mhz)δ=8.39(d,j=9.1hz,1h),8.07(s,1h),8.03–8.00(m,2h),7.88(d,j=9.0hz,1h),7.63(dd,j=9.2,2.0hz,1h),7.19–7.14(m,3h),7.14–7.10(m,2h),2.68(s,3h).13cnmr(150mhz)δ=139.3,134.7,132.3,131.8,131.4,130.5,129.9,129.7,127.4,119.9,119.2,117.0,39.9.hr-ms(esi)m/zcalcdfor:c17h13brno4s80se-[m-h-]453.9034,found453.9021.
n-(2-((4-fluorophenyl)selanyl)-4-methylphenyl)methanesulfonamide:1hnmr(600mhz)δ=7.58(d,j=8.3hz,1h),7.52(d,j=1.6hz,1h),7.32–7.28(m,2h),7.25–7.20(m,2h),6.99–6.96(m,2h),2.71(s,3h),2.33(s,3h).13cnmr(150mhz)δ=162.5(d,j=247.9hz),138.1,136.1,135.8,133.2(d,j=8.1hz),132.0,124.8,121.2,120.3,117.1(d,j=21.8hz),39.2,20.7.hr-ms(esi)m/zcalcdfor:c14h13fno2s80se-[m-h-]357.9831,found357.9822.
n-(2-((4-chlorophenyl)selanyl)-4-methylphenyl)methanesulfonamide:1hnmr(600mhz)δ=7.60(d,j=8.3hz,1h),7.54(s,1h),7.28-7.24(m,1h),7.24–7.18(m,5h),2.73(s,3h),2.34(s,3h).13cnmr(150mhz)δ=138.6,136.4,135.8,133.8,132.3,132.0,129.9,128.7,120.2,120.1,39.3,20.7.hr-ms(esi)m/zcalcdfor:c14h13clno2s80se-[m-h-]373.9534,found373.9526.
n-(2-((4-bromophenyl)selanyl)-4-methylphenyl)methanesulfonamide:1hnmr(600mhz)δ=7.60(d,j=8.1hz,1h),7.54(s,1h),7.36(d,j=8.3hz,2h),7.32–7.18(m,2h),7.13(d,j=6.8hz,2h),2.73(s,3h),2.34(s,3h).13cnmr(150mhz)δ=138.6,136.4,135.8,132.8,132.7,132.4,132.2,129.5,121.7,120.1,39.3,20.7.hr-ms(esi)m/zcalcdfor:c14h13brno2s80se-[m-h-]417.9032,found417.9021.
n-(4-methyl-2-((4-(trifluoromethyl)phenyl)selanyl)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.65(d,j=8.3hz,1h),7.58(d,j=1.6hz,1h),7.48(d,j=8.3hz,2h),7.32–7.28(m,3h),7.24(s,1h),2.79(s,3h),2.35(s,3h).13cnmr(150mhz)δ=139.2,136.9,136.1,135.9,132.9,129.7,129.4(q,j=32.7hz),126.4(q,j=3.0hz),123.9(q,j=272.0hz),119.9,118.5,39.5,20.6.hr-ms(esi)m/zcalcdfor:c15h13f3no2s80se-[m-h-]407.9799,found407.9790.
n-(2-((3-chlorophenyl)selanyl)-4-methylphenyl)methanesulfonamide:1hnmr(600mhz)δ=7.64(d,j=8.3hz,1h),7.57(d,j=1.7hz,1h),7.28(dd,j=8.3,1.7hz,1h),7.23–7.16(m,4h),7.14–7.12(m,1h),2.74(s,3h),2.35(s,3h).13cnmr(150mhz)δ=139.0,136.6,135.8,135.6,132.6,132.4,130.7,129.8,128.2,127.6,119.9,119.2,39.3,20.7.hr-ms(esi)m/zcalcdfor:c14h13clno2s80se-[m-h-]373.9534,found373.9526.
n-(4-methyl-2-(p-tolylthio)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.59(d,j=8.3hz,1h),7.40(d,j=1.6hz,1h),7.23–7.20(m,2h),7.10–7.04(m,4h),2.67(s,3h),2.33(s,3h),2.30(s,3h).13cnmr(150mhz)δ=137.2,136.9,136.1,135.5,131.7,131.5,130.4,128.9,123.6,120.6,39.0,21.1,20.7.hr-ms(esi)m/zcalcdfor:c15h16no2s2-[m-h-]306.0639,found306.0628.
n-(4-methoxy-2-(p-tolylthio)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.59(d,j=8.9hz,1h),7.15–7.10(m,4h),7.00(d,j=2.8hz,1h),6.91(dd,j=8.9,2.8hz,1h),6.86(s,1h),3.77(s,3h),2.71(s,3h),2.32(s,3h).13cnmr(150mhz)δ=157.5,137.9,130.6,130.5,130.4,130.0,127.7,124.2,119.9,115.6,55.7,39.1,21.1.hr-ms(esi)m/zcalcdfor:c15h16no3s2-[m-h-]322.0587,found322.0577.
n-(2-(p-tolylthio)-4-(trifluoromethyl)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.84(d,j=1.7hz,1h),7.79(d,j=8.6hz,1h),7.63(dd,j=8.7,1.7hz,1h),7.56(s,1h),7.15–7.06(m,4h),2.74(s,3h),2.30(s,3h).13cnmr(150mhz)δ=141.6,138.2,133.3(q,j=3.0hz),130.7,129.9,129.6,127.9(q,j=3.3hz),127.0(q,j=33.3hz),124.0,123.5(q,j=272.3hz),118.8,39.8,21.1.hr-ms(esi)m/zcalcdfor:c15h13f3no2s2-[m-h-]360.0358,found360.0345.
n-(4-chloro-2-(p-tolylthio)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.62(d,j=8.7hz,1h),7.49(d,j=2.4hz,1h),7.34(dd,j=8.8,2.4hz,1h),7.21(s,1h),7.14–7.10(m,4h),2.72(s,3h),2.32(s,3h).13cnmr(150mhz)δ=138.3,136.6,134.8,132.6,130.7,130.5,130.1,129.9,126.6,121.8,39.5,21.2.hr-ms(esi)m/zcalcdfor:c14h13clno2s2-[m-h-]326.0093,found326.0082.
n-(5-chloro-2-(p-tolylthio)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.73(d,j=2.1hz,1h),7.53(d,j=8.3hz,1h),7.42(s,1h),7.14(dd,j=8.3,2.1hz,1h),7.10(d,j=8.2hz,2h),7.07–7.04(m,2h),2.72(s,3h),2.30(s,3h).13cnmr(150mhz)δ=139.7,137.7,137.5,137.1,130.9,130.5,129.0,125.3,121.5,119.5,39.5,21.1.hr-ms(esi)m/zcalcdfor:c14h13clno2s2-[m-h-]326.0093,found326.0082.
n-(6-(p-tolylthio)benzo[d][1,3]dioxol-5-yl)methanesulfonamide:1hnmr(600mhz)δ=7.28(s,1h),7.24(s,1h),7.08(d,j=8.2hz,2h),7.05(s,1h),7.04(d,j=8.2hz,2h),6.03(s,2h),2.66(s,3h),2.30(s,3h).13cnmr(150mhz)δ=150.1,145.3,137.1,134.0,132.2,130.3,128.2,115.5,115.2,103.1,102.2,39.0,21.1.hr-ms(esi)m/zcalcdfor:c15h14no4s2-[m-h-]336.0380,found336.0370.
n-(4-methyl-2-(phenylthio)phenyl)methanesulfonamide:1hnmr(600mhz)δ=7.62(d,j=8.3hz,1h),7.45(d,j=1.6hz,1h),7.27(d,j=7.6hz,2h),7.26–7.25(m,2h),7.21–7.18(m,1h),7.13(t,j=1.6hz,1h),7.11(d,j=0.9hz,1h),2.67(s,3h),2.34(s,3h).13cnmr(150mhz)δ=137.5,136.6,135.5,132.1,129.6,128.1,126.9,122.3,120.4,39.1,20.7.hr-ms(esi)m/zcalcdfor:c14h14no2s2-[m-h-]292.0482,found292.0471.
n-(2-((4-methoxyphenyl)thio)-4-methylphenyl)methanesulfonamide:1hnmr(600mhz)δ=7.55(d,j=8.3hz,1h),7.32(d,j=1.3hz,1h),7.21–7.15(m,4h),6.86–6.82(m,2h),3.78(s,3h),2.70(s,3h),2.31(s,3h).13cnmr(150mhz)δ=159.5,135.7,135.6,135.3,131.8,131.1,125.4,125.0,121.0,115.3,55.5,39.1,20.8.hr-ms(esi)m/zcalcdfor:c15h16no3s2-[m-h-]322.0587,found322.0577.
n-(2-((4-chlorophenyl)thio)-4-methylphenyl)methanesulfonamide:1hnmr(600mhz)δ=7.61(d,j=8.3hz,1h),7.41(d,j=1.7hz,1h),7.29–7.27(m,1h),7.25–7.21(m,3h),7.05–7.02(m,2h),2.79(s,3h),2.34(s,3h).13cnmr(150mhz)δ=137.4,136.5,135.7,134.0,132.9,132.4,129.7,129.1,121.7,120.3,39.4,20.7.hr-ms(esi)m/zcalcdfor:c14h13clno2s2-[m-h-]326.0094,found326.0082.
经检测该产品的苯环上对位或者间位含有吸电子基团或供电子基团例如:f,cl,cf3,me,et,ome等,硒化反应进行的较为顺利,得到产物产率在45-90%之间;磺酰基部分不同取代的底物能以52-88%收率的到相应硒化产物;不同取代的磺酰芳胺底物与芳基硫醚反应,以44-95%收率得到硫化产物。
得到的硒化的f,cl,br等产物可以发生偶联反应,进行更复杂的化合物的制备;给电子基团例如甲基,甲氧基和乙氧基等取代的磺酰芳胺硫化产率较高,最高达到95%。
产物结构均经过核磁共振氢谱,碳谱和高分辨确认得到上述方法制备的硒化产物可以达到25个,硫化产物为9个。
- 还没有人留言评论。精彩留言会获得点赞!
- 新型磺酰亚胺盐化合物、其制造方法和包含其的光产酸剂以及感光性树脂组合物与制造工艺
- (R)‑3,5‑双(三氟甲基)苯乙醇的生物制备方法与制造工艺
- 一种五氟磺草胺和丙炔噁草酮复配可分散油悬浮剂及其制备方法与制造工艺
- 多孔性酰亚胺系树脂膜制造系统、隔膜、及多孔性酰亚胺系树脂膜制造方法与制造工艺
- 含酰亚胺结构的环三磷腈无卤阻燃剂、制备方法及用途与制造工艺
- 一种苝酰亚胺类化合物及其制备方法和应用与制造工艺
- 双芘修饰苝酰亚胺衍生物荧光探针及其合成方法和应用与制造工艺
- 一种耐高温表面自润滑的聚酰胺酰亚胺绝缘漆的制造方法与工艺
- 一种耐高温表面自润滑的聚酰胺酰亚胺绝缘漆的制造方法与工艺
- 一种无溶剂研磨法制备双苯磺酰亚胺的方法与制造工艺