1-磷杂降冰片烯类手性膦催化剂、其合成方法及应用
本发明涉及本发明属于有机化学合成领域,具体涉及一种在手性膦催化的不对称合成领域应用的1-磷杂降冰片烯类有机膦催化剂、其合成方法及应用。
背景技术:
在不对称催化领域,手性有机膦化物是重要有机催化剂。由于拥有一对孤对电子,三价磷化物可以直接作为亲核催化剂,进攻缺电子烯烃生成碳负离子中间体,进而催化化学反应。在手性膦催化剂的发展过程中,化学家往往把关注点放在了对于手性骨架的选择上,对于具有磷手性中心亲核膦催化剂的合成关注较少。磷本身的性质导致了磷手性中心难以合成而且高温下易于外消旋化,所以造成磷手性中心配体的研究非常少。然而有机膦小分子催化反应中反应的活性和对映选择性往往依赖于有机膦催化剂的电性、酸碱性和位阻,因此有机催化剂的微小变化常常能够影响催化反应的决速步骤。磷手性中心催化剂可以通过改变磷原子上取代基来改变催化剂的电子效应和位阻。并且这类催化剂的手性位点直接与反应底物相连,这就增加了手性诱导和传递的可能性。磷手性中心催化剂的这些优势促使化学家努力设计合成新型的磷手性催化剂,旨在克服制约其发展的其合成步骤繁琐且易于消旋的缺点,设计合成新型的磷手性催化剂。因为磷本身的性质导致了磷手性中心难以合成而且高温下易于外消旋化,所以造成磷手性中心配体的研究非常少。
到目前为止,将磷原子嵌入到刚性骨架中设计出具有磷手性中心手性有机膦催化剂仍然未见报道。
技术实现要素:
针对目前的技术现状,本发明的目的之一在于提供一种1-磷杂降冰片烯类手性膦催化剂。其结构如下式iii和iii’所示:
式中,ar为苯基、取代苯基、萘基、取代萘基;优选的,ar为苯基、单取代苯基、萘基;更优选的,ar为苯基、4-单取代苯基、2-萘基;更进一步优选的,ar为苯基、4-甲基苯基、4-甲氧基苯基、4-苄基苯基、4-叔丁基苯基、4-叔丁氧基苯基苯基、2-萘基。
作为本发明的优选方案,所述1-磷杂降冰片烯类手性膦催化剂的结构如下所示:
本发明的目的之二在于提供所述催化剂的合成方法,所述方法以磷杂环戊二烯类化合物为中间产物,通过h[1,5]迁移和磷杂d-a反应生成1-磷杂降冰片烯类手性膦催化剂;所述反应式如下所示:
式中,ar为苯基、取代苯基、萘基、取代萘基;优选的,ar为苯基、单取代苯基、萘基;更优选的,ar为苯基、4-单取代苯基、2-萘基;更进一步优选的,ar为苯基、4-甲基苯基、4-甲氧基苯基、4-苄基苯基、4-叔丁基苯基、4-叔丁氧基苯基苯基、2-萘基。
作为本发明的优选方案,所述反应包括如下步骤:
步骤一)分别取i化合物原料于一定容积的schlenk瓶中,惰性气体氛围下加入无水有机溶剂,低温下加入芳基格氏试剂,然后在此温度下继续反应至反应完全,淬灭反应,得到相应的产物ii-a~ii’-g;反应式如下:
步骤二)使用一定容积的schlenk瓶,惰性气体氛围下,加入加氢试剂,洗涤;然后加入ii化合物原料的无水四氢呋喃溶液;搅拌至反应完全,得到iii-a~iii’-g;其反应式如下所示:
作为本发明的优选方案,所述反应包括如下步骤:
步骤一)分别取i化合物原料于schlenk瓶中,抽真空,充入氮气三次,加入无水四氢呋喃,在-78℃的条件下缓慢加入2.0当量的芳基格式试剂,然后在此温度下继续反应10h。tlc检测反应进程,当反应完全,向反应体系加入nh4cl溶液,然后使用乙酸乙酯萃取,合并有机层,使用mgso4干燥,过滤,减压蒸去溶剂,柱层析分离;得到相应的产物ii-a~ii’-g,产率39-46%;
步骤二)使用50ml的schlenk瓶,抽真空,充入氮气三次,加入兰尼镍,使用无水乙醇洗涤三次,然后使用无水四氢呋喃洗涤三次。最后加入ii化合物原料的无水四氢呋喃溶液;搅拌5小时,tlc检测反应,当反应完全,柱层析分离得到iii-a~iii’-g,产率71-87%。
本发明的另一目的在于提供了所述催化剂及其中间体的应用。具体是,以烯基羟吲哚衍生物作为缺电子的烯烃,实现了有机膦的基于取代的联烯酸酯的(4+2)环加成反应。优选的,所述催化剂为ii-d;其反应式如下所示:
本发明还有一目的在于提供一种螺环化合物p1,所述螺环化合物p1结构如下,
本发明提供选用烯基羟吲哚衍生物作为缺电子的烯烃,实现了有机膦催化的基于取代的联烯酸酯的(4+2)环加成反应。克服了使用传统的手性有机催化剂时,高温下无法实现有效的立体诱导的缺陷。
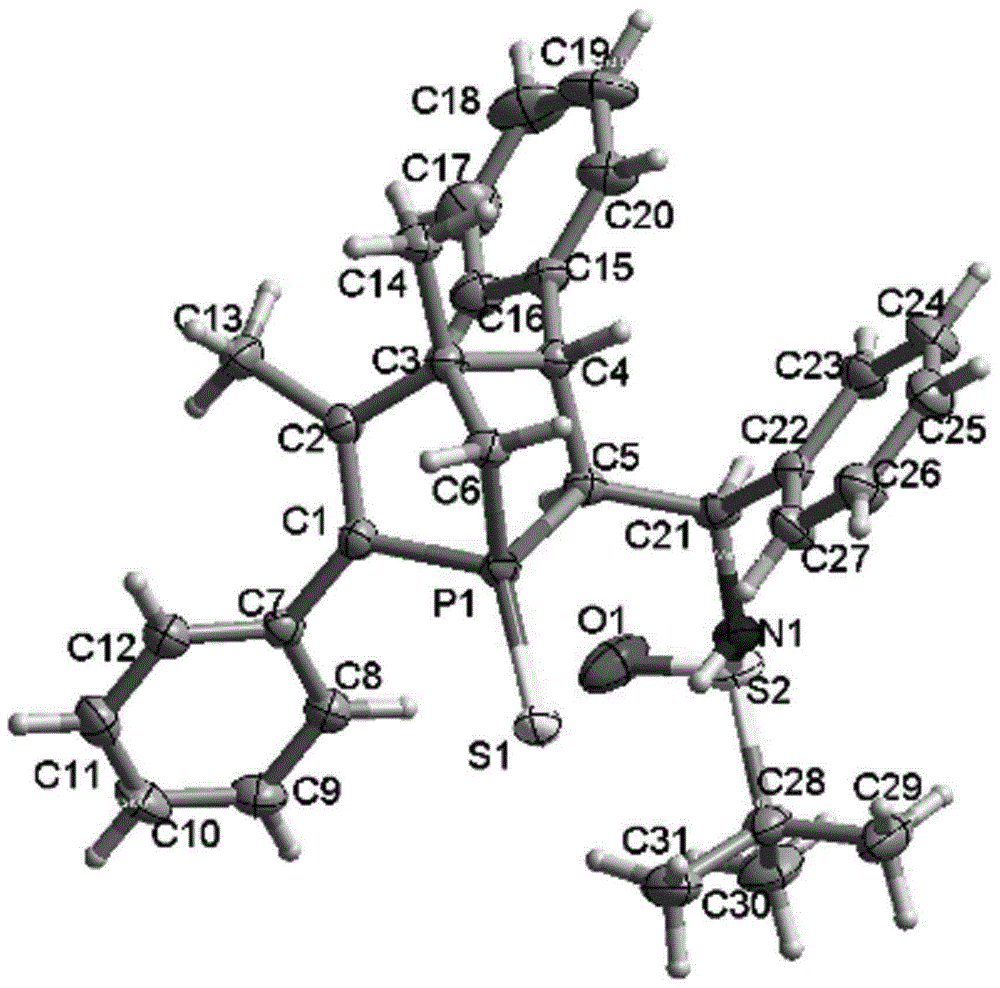
本发明实验过程中,通过对催化剂,溶剂,催化剂的当量和反应温度等条件的优化,发现使用ii-d作为配体时效果最好(化合物ii-d的x-rayx-raycrystalstructure如附图1所示)。通过对反应底物上各个位置取代基的改变,进一步研究此类反应的底物适用性,能够以很高的产率(99%以上),非对映选择性(大于20:1)以及立体选择性(大于99%)得到相应的螺环化合物p1,其结构如下,(化合物p1的x-rayx-raycrystalstructure如附图2所示)。
有益效果:
1、本发明提供的催化剂作为一类结构新颖的手性膦催化剂,通过刚性骨架的引入,形成三个完全束缚的p-c键,成功的解决了磷手性中心易于消旋化的科学难题,此类磷手性催化剂能够广泛的应用到手性膦催化的不对称环化等不对称反应中,具有广阔的商业应用前景。2、本发明提供的合成方法简单、可行性好,收率较高。
附图说明
附图1:化合物ii-d的x-rayx-raycrystalstructure图;
附图2:螺环产物p1化合物p1的x-rayx-raycrystalstructure图。
具体实施方式
为对本发明进行更好地说明,举实施例下:以下所用原料均为市售品。
实施例1化合物i的合成方法,步骤如下:
在n2环境下,将40ml甲醇,肉桂醛(20mmol),原甲酸三甲酯(40mmol)加入100mlschlenk瓶中,然后加入催化剂对甲苯磺酸(10mol%),转入65℃油浴回流10h。反应完全后,加入少量naoh固体,继续搅拌30min。蒸去溶剂,过中性氧化铝干柱,旋干后得化合物a2,产率89%。
在n2环境下,将5ml甲苯,化合物a2(5.6mol),phoshole(5.3mmol)加入75mlschlenk瓶中。140℃油浴中反应2h。然后加入两当量的硫粉,过中性氧化铝柱(pe:dcm=10:1),得到化合物,产率52%。
在n2环境下,将20ml二氯甲烷,上步所得的化合物(10mmol),三氟乙酸(20mmol)加入100mlschlenk瓶中,室温搅拌3h。反应完全后用h2o洗涤(20ml*3),mgso4干燥。蒸干溶剂得化合物a4,产率94%。
whitesolid.31pnmr(121mhz,cdcl3)δ55.74(s)ppm.1hnmr(300mhz,cdcl3)δ1.41(s,3h,ch3),1.48(d,j=2.6hz,3h,ch3),2.14–2.29(m,2h,ch2),3.80–3.87(m,1h,ch),3.94(d,j=5.8hz,1h,ch),7.08–7.11(m,2h,ch),7.28–7.32(m,3h,ch),7.40–7.44(m,1h,ch),7.50–7.52(m,4h,ch),10.07(d,j=1.4hz,1h,cho)ppm.13cnmr(75mhz,cdcl3)δ16.3(d,j=12.8hz,ch3),19.6(d,j=17.3hz,ch3),50.3(d,j=19.5hz,c),51.6(s,ch),52.4(d,j=57.0hz,ch2),58.3(d,j=30.0hz,ch),127.8(s,ch),128.1(d,j=1.5hz,ch),128.2(s,2ch),128.5(s,2ch),128.6(s,2ch),129.3(d,j=6.0hz,2ch),131.7(d,j=10.5hz,c),133.9(d,j=67.5hz,c),137.6(d,j=6.0hz,c),157.7(d,j=15.0hz,c),198.9(s,cho)ppm.
在n2环境下,将40mlthf,化合物a4(7.4mmol),r-叔丁基磺酸亚胺(8mmol),四异丙氧基钛(16mmol)加入100mlschlenk瓶中,在50℃的条件下,油浴反应3h。用乙酸乙酯萃取(100ml*3),mgso4干燥。柱层析法分离(dcm:ea=1:1)。得化合物i,产率80%。
yellowsolid.31pnmr(121mhz,cdcl3)δ57.68(s)ppm.1hnmr(300mhz,cdcl3)δ1.23(d,j=13.3hz,9h,3ch3),1.39(s,3h,ch3),1.53(dd,j=4.3,2.6hz,3h,ch3),2.24–2.39(m,2h,ch2),3.68–3.87(m,2h,ch),7.08–7.12(m,2h,ch),7.28–7.31(m,3h,ch),7.36–7.43(m,1h,ch),7.49–7.50(m,4h,ch),8.23–8.34(m,1h,ch)ppm.13cnmr(75mhz,cdcl3)δ16.3(d,j=12.0hz,ch3),19.8(d,j=17.3hz,ch3),22.7(d,j=17.3hz,3ch3),50.6(dd,j=19.5,10.5hz,c),52.8(dd,j=61.5,35.3hz,ch),52.8(dd,j=55.5,11.3hz,ch2),56.3(d,j=9.0hz,ch),57.5(dd,j=28.5,2.3hz,c),127.8(d,j=3.0hz,ch),128.0(s,,ch),128.3(d,j=1.5hz,2ch),128.5(d,j=2.3hz,2ch),128.5(s,2ch),129.3(dd,j=5.3,1.5hz,2ch),131.9(dd,j=10.5,2.3hz,c),134.6(dd,j=68.3,9.0hz,c),137.4–137.6(m,c),156.7(dd,j=14.3,9.0hz,c),165.8–166.6(m,ch)ppm.
实施例2化合物ii-a至ii’-g的合成方法,步骤如下:
分别取i化合物原料于50mlschlenk瓶中,抽真空,充入氮气三次,加入无水四氢呋喃,在-78℃的条件下加入2.0当量的芳基格式试剂,然后再此温度下继续反应10h,当反应完全,向反应体系加入nh4cl溶液,然后使用乙酸乙酯萃取,合并有机层,使用mgso4干燥,过滤,减压蒸去溶剂,柱层析分离。得到相应的产物ii-a~ii’-g。产率:39-46%
代表性核磁数据ii-e和ii’-e:
ii-d:whitesolid.31pnmr(121mhz,cdcl3)δ53.39(s)ppm.1hnmr(300mhz,cdcl3)δ1.11(s,3h,ch3),1.27(s,9h,3ch3),1.29(s,9h,3ch3),1.48(d,j=2.4hz,3h,ch3),1.54–1.60(m,1h,ch2),1.98(t,j=10.3hz,1h,ch2),2.95(d,j=7.1hz,1h,ch),3.00–3.08(m,1h,ch),4.76(ddd,j=21.9,11.7,4.5hz,1h,ch),5.88(d,j=9.1hz,1h,nh),7.01–7.04(m,2h,ch),7.24–7.27(m,3h,ch),7.33–7.35(m,2h,ch),7.38–7.51(m,5h,ch),7.57(d,j=8.2hz,2h,ch)ppm.13cnmr(75mhz,cdcl3)δ16.3(d,j=12.0hz,ch3),19.8(d,j=16.5hz,ch3),22.9(s,3ch3),31.3(s,3ch3),34.5(s,c),50.3(d,j=18.8hz,c),52.1(d,j=26.3hz,ch),52.7(d,j=37.5hz,ch2),54.3(d,j=2.3hz,ch),56.7(s,c),61.3(d,j=3.0hz,ch),125.3(s,2ch),127.6(s,ch),127.8(s,ch),128.4(s,2ch),128.5(s,6ch),129.1(d,j=6.0hz,2ch),132.4(d,j=10.5hz,c),135.9(d,j=69.8hz,c),136.1(d,j=4.5hz,c),137.7(d,j=9.8hz,c),150.7(s,c),155.9(d,j=13.5hz,c)ppm.
ii’-d:whitesolid.31pnmr(121mhz,cdcl3)δ52.90(s)ppm.1hnmr(300mhz,cdcl3)δ1.09(s,3h,ch3),1.26(s,9h,3ch3),1.32–1.39(m,1h,ch2),1.34(s,9h,3ch3),1.44(d,j=2.5hz,3h,ch3),1.90–1.98(m,1h,ch2),2.90(d,j=7.2hz,1h,ch),3.53–3.60(m,1h,ch),4.79(ddd,j=23.6,11.3,5.2hz,1h,ch),6.34(d,j=8.7hz,1h,nh),7.06–7.09(m,2h,ch),7.22–7.30(m,3h,ch),7.33–7.39(m,1h,ch),7.41(d,j=8.3hz,2h,ch),7.47–7.49(m,4h,ch),7.61(d,j=8.3hz,2h,ch)ppm.13cnmr(75mhz,cdcl3)δ16.3(d,j=12.0hz,ch3),19.9(d,j=16.5hz,ch3),23.1(s,3ch3),31.4(s,3ch3),34.6(s,c),50.3(d,j=19.5hz,c),51.7(d,j=42.0hz,ch),52.4(d,j=53.3hz,ch2),55.2(d,j=4.5hz,ch),56.2(s,c),61.6(d,j=3.8hz,ch),125.1(s,2ch),127.6(s,ch),127.7(s,ch),128.5(s,4ch),128.6(s,2ch),128.9(s,2ch),129.3(d,j=6.0hz,2ch),132.3(d,j=9.8hz,c),136.0(d,j=3.8hz,c),137.0(s,c)137.3(d,j=9.0hz,c),150.8(s,c),155.5(d,j=13.5hz,c)ppm.
使用50ml的schlenk瓶,抽真空,充入氮气三次,加入兰尼镍,使用无水乙醇洗涤三次,然后使用无水四氢呋喃洗涤三次。最后加入ii化合物原料的无水四氢呋喃溶液。搅拌5小时,tlc检测反应,当反应完全,柱层析分离得到iii-a~iii’-g。产率:71-87%
代表性核磁数据:
(r)-n-((1r)-((1r,2r,4s)-4,5-dimethyl-3,6-diphenyl-1-phosphabicyclo[2.2.1]hept-5-en-2-yl)(phenyl)methyl)-2-methylpropane-2-sulfinamideiii-a.
whitesolid.mp:145-146℃.[α]d=-194(c=0.1,ch2cl2,19℃).31pnmr(121mhz,cdcl3)δ-20.06(s)ppm.1hnmr(300mhz,cdcl3)δ0.80–0.87(m,1h,ch2),1.13(s,9h,3ch3),1.42–1.47(m,1h,ch2),1.47(s,3h,ch3),2.69(d,j=7.2hz,1h,ch),2.78(t,j=6.8hz,1h,ch),3.64(d,j=4.8hz,1h,nh),4.53–4.60(m,1h,ch),7.08–7.11(m,2h,ch),7.23–7.46(m,13h,ch)ppm.13cnmr(75mhz,cdcl3)δ16.4(s,ch3),20.7(s,ch3),22.6(s,3ch3),48.0(d,j=4.5hz,ch2),54.7(d,j=18.7hz,ch),55.0(d,j=2.3hz,ch),56.2(s,c),61.6(d,j=12.0hz,ch),64.4(d,j=5.3hz,c),126.4(s,ch),126.9(s,ch),128.0(s,ch),128.1(s,ch),128.1(s,3ch),128.2(s,2ch),128.4(s,ch),128.5(s,ch),128.6(s,2ch),128.8(s,2ch),138.6(d,j=21.0hz,c),141.0(s,c),141.1(d,j=1.5hz,c),141.6(d,j=21.0hz,c),153.0(s,c)ppm.hrms(esi)m/z:[m+h]+calcdforc31h37nops502.2328;found502.2307.
(r)-n-((1s)-((1s,3s,4r)-4,5-dimethyl-3,6-diphenyl-1-phosphabicyclo[2.2.1]hept-5-en-2-yl)(phenyl)methyl)-2-methylpropane-2-sulfinamideiii'-a.
whitesolid.mp:162-163℃.[α]d=43(c=0.1,ch2cl2,24℃).31pnmr(121mhz,cdcl3)δ-18.63(s)ppm.1hnmr(300mhz,cdcl3)δ0.88(s,9h,3ch3),1.27(s,3h,ch3),1.49(s,3h,ch3),1.50–1.62(m,2h,ch2),2.63(dd,j=10.2,6.5hz,1h,ch),2.89(d,j=6.4hz,1h,ch),3.65(s,1h,nh),4.55(dd,j=10.2,4.4hz,1h,ch),7.2–7.4(m,15h,ch)ppm.13cnmr(75mhz,cdcl3)δ16.4(s,ch3),20.6(s,ch3),22.3(s,3ch3),48.2(d,j=5.3hz,ch2),55.3(s,c),56.3(d,j=18.0hz,ch),57.9(d,j=2.3hz,ch),64.3(d,j=17.3hz,ch),65.5(d,j=5.3hz,c),126.4(s,ch),127.2(s,ch),128.0(s,ch),128.2(s,ch),128.3(s,3ch),128.4(s,ch),128.4(s,3ch),128.4(s,2ch),129.2(s,2ch),138.5(d,j=21.0hz,c),141.2(s,c),141.7(d,j=2.3hz,c),141.9(s,c),152.7(s,c)ppm.hrms(esi)m/z:[m+h]+calcdforc31h37nops502.2328;found502.2289.
(r)-n-((1r)-((1r,2r,4s)-4,5-dimethyl-3,6-diphenyl-1-phosphabicyclo[2.2.1]hept-5-en-2-yl)(p-tolyl)methyl)-2-methylpropane-2-sulfinamideiii-b.
whitesolid.mp:179-180℃.[α]d=-155(c=0.1,ch2cl2,18℃).31pnmr(121mhz,cdcl3)δ-20.39(s)ppm.1hnmr(300mhz,cdcl3)δ0.84–0.91(m,1h,ch2),1.12(s,9h,3ch3),1.15–1.16(m,3h,ch3),1.43–1.48(m,1h,ch2),1.48(s,3h,ch3),2.38(s,3h,ch3),2.70(d,j=7.2hz,1h,ch),2.78(t,j=6.8hz,1h,ch),3.59(d,j=4.8hz,1h,nh),4.49–4.57(m,1h,ch),7.09–7.15(m,2h,ch),7.17–7.39(m,12h,ch)ppm.13cnmr(75mhz,cdcl3)δ16.4(s,ch3),20.7(s,ch3),21.3(s,ch3),22.6(s,3ch3),48.1(d,j=5.3hz,ch2),54.7(d,j=18.8hz,ch),55.2(d,j=2.3hz,ch),56.1(s,c),61.5(d,j=12.0hz,ch),64.4(d,j=12.8hz,c),126.4(s,ch),126.8(s,ch),127.9(d,j=4.5hz,2ch),128.1(s,2ch),128.2(s,2ch),128.4(s,ch),128.5(s,ch),128.8(s,2ch),129.3(s,2ch),137.8(s,c),138.1(d,j=2.3hz,c),138.6(d,j=20.3hz,c),141.1(s,c),141.7(d,j=15.8hz,c),153.0(s,c)ppm.hrms(esi)m/z:[m+na]+calcdforc32h38nopsna538.2304;found538.2294.
(r)-n-((1s)-((1s,3s,4r)-4,5-dimethyl-3,6-diphenyl-1-phosphabicyclo[2.2.1]hept-5-en-2-yl)(p-tolyl)methyl)-2-methylpropane-2-sulfinamideiii'-b.
whitesolid.mp:176-177℃.[α]d=35(c=0.1,ch2cl2,26℃).31pnmr(121mhz,cdcl3)δ-18.59(s)ppm.1hnmr(300mhz,cdcl3)δ0.89(s,9h,3ch3),1.28(s,3h,ch3),1.49(s,3h,ch3),1.55–1.62(m,2h,ch2),2.37(s,3h,ch3),2.54(dd,j=10.2,6.5hz,1h,ch),2.90(d,j=6.4hz,1h,ch),3.67(s,1h,nh),4.53(dd,j=10.3,4.4hz,1h,ch),7.18–7.37(m,14h,ch)ppm.13cnmr(75mhz,cdcl3)δ16.4(s,ch3),20.6(s,ch3),21.4(s,ch3),22.3(s,3ch3),48.2(d,j=6.0hz,ch2),55.3(s,c),56.4(d,j=17.3hz,ch),57.9(d,j=2.3hz,ch),64.1(d,j=17.3hz,ch),65.5(d,j=5.3hz,c),126.4(s,ch),127.2(s,ch),128.2(s,ch),128.2(s,ch),128.3(s,4ch),128.4(s,2ch),129.2(s,4ch),137.6(s,c),138.6(d,j=21.7hz,c),138.6(d,j=3.0hz,c),141.3(s,c),141.9(d,j=17.3hz,c),152.7(s,c)ppm.hrms(esi)m/z:[m+na]+calcdforc32h38nopsna538.2304;found538.2291.
(r)-n-((1r)-[1,1'-biphenyl]-4-yl((1r,2r,4s)-4,5-dimethyl-3,6-diphenyl-1-phosphabicyclo[2.2.1]hept-5-en-2-yl)methyl)-2-methylpropane-2-sulfinamideiii-c.
whitesolid.mp:82-83℃.[α]d=-155(c=0.1,ch2cl2,20℃).31pnmr(121mhz,cdcl3)δ-19.61(s)ppm.1hnmr(300mhz,cdcl3)0.94–1.01(m,1h,ch2),1.16(s,9h,3ch3),1.17(s,3h,ch3),1.48–1.54(m,1h,ch2),1.50(s,3h,ch3),2.75(d,j=7.2hz,1h,ch),2.83(t,j=6.9hz,1h,ch),3.69(d,j=5.2hz,1h,nh),4.60–4.67(m,1h,ch),7.11–7.14(m,2h,ch),7.25–7.32(m,4h,ch),7.36–7.41(m,5h,ch),7.45–7.53(m,4h,ch),7.63–7.66(m,4h,ch)ppm.13cnmr(75mhz,cdcl3)δ16.4(s,ch3),20.7(s,ch3),22.6(s,3ch3),48.2(d,j=4.5hz,ch2),54.8(d,j=18.7hz,ch),55.2(d,j=1.5hz,ch),56.3(s,c),61.6(d,j=12.0hz,ch),64.5(d,j=5.3hz,c),126.4(s,ch),126.9(s,ch),127.1(s,2ch),127.2(s,2ch),127.4(s,ch),128.2(s,2ch),128.3(s,2ch),128.4(s,2ch),128.5(s,ch),128.5(s,ch),128.8(s,2ch),128.8(s,2ch),138.6(d,j=21.0hz,c),140.2(d,j=2.3hz,c),140.6(s,c),140.8(s,c),141.0(s,c),141.7(d,j=15.8hz,c),153.1(s,c)ppm.hrms(esi)m/z:[m+h]+calcdforc37h41nops578.2641;found578.2651.
(r)-n-((1s)-[1,1'-biphenyl]-4-yl((1s,3s,4r)-4,5-dimethyl-3,6-diphenyl-1-phosphabicyclo[2.2.1]hept-5-en-2-yl)methyl)-2-methylpropane-2-sulfinamideiii'-c.
whitesolid.mp:186-187℃.[α]d=38(c=0.1,ch2cl2,26℃).31pnmr(121mhz,cdcl3)δ-18.76(s)ppm.1hnmr(300mhz,cdcl3)δ0.90(s,9h,3ch3),1.29(s,3h,ch3),1.50(s,3h,ch3),1.58–1.68(m,2h,ch2),2.56(dd,j=10.2,6.4hz,1h,ch),2.92(d,j=6.4hz,1h,ch),3.69(s,1h,nh),4.60(dd,j=10.3,4.6hz,1h,ch),7.18–7.37(m,11h,ch),7.43–7.48(m,4h,ch),7.61–7.67(m,4h,ch)ppm.13cnmr(75mhz,cdcl3)δ16.4(s,ch3),20.6(s,ch3),22.3(s,3ch3),48.2(d,j=5.3hz,ch2),55.4(s,c),56.2(d,j=18.0hz,ch),57.9(s,ch),64.0(d,j=17.3hz,ch),65.5(d,j=4.5hz,c),126.4(s,ch),127.0(s,2ch),127.1(s,2ch),127.2(s,ch),127.3(s,ch),128.2(s,ch),128.3(s,3ch),128.4(s,2ch),128.7(s,2ch),128.8(s,ch),128.8(s,ch),129.2(s,2ch),138.5(d,j=21.0hz,c),140.6(s,c),140.7(s,c),140.8(s,c),141.2(s,c),141.8(d,j=16.5hz,c),152.8(s,c)ppm.hrms(esi)m/z:[m+h]+calcdforc37h41nops578.2641;found578.2635.
(r)-n-((1r)-(4-(tert-butyl)phenyl)((1r,2r,4s)-4,5-dimethyl-3,6-diphenyl-1-phosphabicyclo[2.2.1]hept-5-en-2-yl)methyl)-2-methylpropane-2-sulfinamideiii-d.
whitesolid.mp:164-165℃.[α]d=-216(c=0.1,ch2cl2,21℃).31pnmr(121mhz,cdcl3)δ-21.49(s)ppm.1hnmr(300mhz,cdcl3)δ0.80(t,j=21.4hz,1h,ch2),1.14(s,3h,ch3),1.16(s,9h,3ch3),1.35(s,9h,3ch3),1.44–1.50(m,1h,ch2),1.49(s,3h,ch3),2.68(d,j=7.3hz,1h,ch),2.81(t,j=6.6hz,1h,ch),3.66(d,j=4.4hz,1h,nh),4.57–4.64(m,1h,ch),7.10(d,j=6.7hz,2h,ch),7.23–7.27(m,4h,ch),7.38–7.42(m,8h,ch)ppm.13cnmr(75mhz,cdcl3)δ16.4(s,ch3),20.8(s,ch3),22.6(s,3ch3),31.4(s,3ch3),34.6(s,c),48.1(d,j=4.5hz,ch2),54.5(s,ch),54.8(s,ch),56.2(s,c),61.0(d,j=10.5hz,ch),64.3(d,j=42.8hz,c),125.4(s,2ch),126.4(s,ch),126.8(s,ch),127.7(d,j=3.8hz,2ch),128.1(s,2ch),128.2(s,2ch),128.5(d,j=7.5hz,2ch),128.8(s,2ch),137.9(d,j=1.5hz,c),138.7(d,j=21.0hz,c),141.0(s,c),141.8(d,j=15.8hz,c),151.0(s,c),152.9(s,c)ppm.hrms(esi)m/z:[m+h]+calcdforc35h45nops558.2954;found558.2956.
n-((1s)-(4-(tert-butyl)phenyl)((1s,3s,4r)-4,5-dimethyl-3,6-diphenyl-1-sulfido-1-phosphabicyclo[2.2.1]hept-5-en-2-yl)methyl)-2-methylpropane-2-sulfinamideiii'-d.
whitesolid.mp:169-170℃.[α]d=10(c=0.1,ch2cl2,25℃).31pnmr(121mhz,cdcl3)δ-18.08(s)ppm.1hnmr(300mhz,cdcl3)δ0.91(s,9h,3ch3),1.27(s,3h,ch3),1.35(s,9h,3ch3),1.49(s,3h,ch3),1.54–1.64(m,2h,ch2),2.51–2.56(m,1h,ch),2.90(d,j=6.4hz,1h,ch),3.65(s,1h,nh),4.55(dd,j=9.7,5.0hz,1h,ch),7.17–7.40(m,14h,ch)ppm.13cnmr(75mhz,cdcl3)δ16.4(s,ch3),20.6(s,ch3),22.4(s,3ch3),31.5(s,3ch3),34.6(s,c),48.2(d,j=4.5hz,ch2),55.3(s,c),56.4(d,j=17.3hz,ch),57.6(d,j=2.3hz,ch),63.6(d,j=17.3hz,ch),65.4(d,j=5.3hz,c),125.2(s,2ch),126.4(s,ch),127.1(s,ch),128.0(d,j=3.0hz,2ch),128.2–128.3(m,4ch),128.4(s,2ch),129.2(s,2ch),138.4(d,j=3.0hz,c),138.7(s,c),141.2(s,c),141.9(d,j=16.5hz,c)150.7(s,c),152.7(s,c)ppm.hrms(esi)m/z:[m+h]+calcdforc35h45nops558.2954;found558.2952.
(1r)-(4-(tert-butyl)phenyl)((1r,2r,4s)-4,5-dimethyl-3,6-diphenyl-1-phosphabicyclo[2.2.1]hept-5-en-2-yl)methanamine5e.
whitesolid.mp:54-55℃.[α]d=-189(c=0.1,ch2cl2,21℃).31pnmr(121mhz,cdcl3)δ-20.83(s)ppm.1hnmr(300mhz,cdcl3)δ1.06(t,j=10.6hz,1h,ch2),1.17(s,3h,ch3),1.32(s,9h,3ch3),1.47–1.53(m,1h,ch2),1.50(s,3h,ch3),1.76(s,2h,nh2),2.56(t,j=6.3hz,1h,ch),2.77(d,j=6.8hz,1h,ch),4.21–4.26(m,1h,ch),7.05(d,j=7.0hz,2h,ch),7.17–7.31(m,8h,ch),7.40–7.41(m,4h,ch)ppm.13cnmr(75mhz,cdcl3)δ16.4(s,ch3),20.7(s,ch3),31.4(s,3ch3),34.4(s,c),48.5(d,j=3.0hz,ch2),54.9(d,j=2.3hz,ch),56.2(d,j=17.3hz,ch),58.3(d,j=12.0hz,ch),64.3(d,j=4.5hz,c),125.0(s,2ch),126.3(s,2ch),126.8(d,j=3.0hz,2ch),127.8(s,2ch),128.3(s,2ch),128.5(s,ch),128.6(s,ch),128.8(s,2ch)138.8(d,j=21.0hz,c),141.5(d,j=3.0hz,c),141.8(s,c),142.0(s,c),149.9(s,c),153.0(s,c)ppm.hrms(esi)m/z:[m+na]+calcdforc31h36npna476.2478;found476.2430.
最后说明的是,以上实施例仅用以说明本发明的技术方案而非限制,本领域普通技术人员对本发明的技术方案所做的其他修改或者等同替换,只要不脱离本发明技术方案的精神和范围,均应涵盖在本发明的权利要求范围当中。
- 还没有人留言评论。精彩留言会获得点赞!