一种N-(2-苯甲酰基苯基)-2-三氟乙酰氨基烷酰胺衍生物的制备方法
一种n-(2-苯甲酰基苯基)-2-三氟乙酰氨基烷酰胺衍生物的制备方法
技术领域
1.本发明属于有机合成领域,具体涉及一种n-(2-苯甲酰基苯基)-2-三氟乙酰氨基烷酰胺衍生物的制备方法。
背景技术:
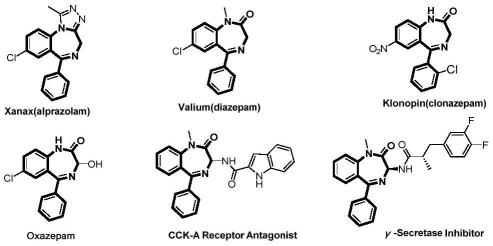
2.苯二氮卓骨架在药物化学中是一种重要的结构,在苯二氮卓家族中,5-芳基-1,4-苯二氮杂卓-2-酮骨架在商业药物中普遍存在,如沙那克(阿普唑仑)、安定(地西泮)和克洛宁(氯硝西泮)。
[0003][0004]
这些药物主要作为gaba受体激动剂用于中枢神经系统临床适应症,如焦虑、失眠、肌肉痉挛、癫痫和惊厥。除了这些经典的适应症外,5-芳基-1,4-苯二氮卓-2-酮骨架还被广泛用于其他生物活性化合物,如胆囊收缩素拮抗剂和γ-分泌酶抑制剂。由于其在药物发现中的广泛应用,开发新的高效合成方法来制备这些药物是非常有意义的。
[0005]
(1)制备5-芳基-1,4-苯二氮杂-2-酮类化合物常用的路线包括以下三步((a)k.hirai,t.ishiba,h.sugimoto,t.fujishita,y.tsukinoki and k.hirose,j.med.chem.1981,24,20-27;(b)r.reddy,f.ballante,n.j.zhou and g.r.marshall,eur j med chem 2017,127,531-553.):官能化2-氨基二芳基甲酮与n-保护氨基酸的缩合反应、脱保护和通过亚胺形成环化,其中缩合反应的反应式如下:
[0006][0007]
该路线涉及到一个关键中间体n-(2-苯甲酰基苯基)-2-氨基烷酰胺衍生物的制备,需要使用到官能化2-氨基二苯甲酮作为起始材料,但是官能化2-氨基二苯甲酮不能直接购买得到,不易获得,限制了该方法的使用。
[0008]
为此,如果能提供一种新的n-(2-苯甲酰基苯基)-2-氨基烷酰胺衍生物的制备方法具有重要的意义。
技术实现要素:
[0009]
本发明提供了一种n-(2-苯甲酰基苯基)-2-三氟乙酰氨基烷酰胺衍生物的制备方法,该制备方法可以采用价廉易得的原料方便地合成n-(2-苯甲酰基苯基)-2-三氟乙酰氨基烷酰胺衍生物。
[0010]
一种n-(2-苯甲酰基苯基)-2-三氟乙酰氨基烷酰胺衍生物的制备方法,包括以下步骤:
[0011]
在钯催化剂和银添加剂的存在下,含有n-三氟乙酰基氨基酸衍生物与碘苯化合物进行芳基化氧化反应,得到苯酰基苯取代的n-三氟乙酰基氨基酸衍生物;
[0012][0013]
其中,r2选自h、c1~c6烷基、c1~c6烷氧基或卤素;
[0014]
r1选自h、c1~c6烷基、c1~c6烷氧基、c1~c6烷氧羰基、氰基、硝基或卤素;
[0015]
r为h、c1~c6烷基或者甲硫基取代的c1~c6烷基。
[0016]
作为优选,r2选自h、甲基、甲氧基、f、cl或br;
[0017]
r1选自h、甲基、甲氧基、甲氧羰基、乙氧羰基、氰基、硝基、f、cl或br;
[0018]
r为h、甲基、1,1环乙基或者甲硫基取代的乙基。
[0019]
作为优选,所述的钯催化剂为醋酸钯;
[0020]
所述的添加剂为碳酸银。
[0021]
作为优选,所述的芳基化氧化反应的溶剂为dmac,反应时还添加分子筛。
[0022]
作为优选,所述的芳基化氧化反应的温度为110~140℃,反应时间为20~30小时
[0023]
本发明中,所述的含有n-三氟乙酰基氨基酸衍生物的制备方法如下:
[0024]
(1)邻甲苯胺衍生物与n-boc-l-氨基酸衍生物在n,n'-二环己基碳二亚胺和4-(二甲氨基)吡啶的作用下在二氯甲烷中进行酰胺化反应,得到含n-boc-l-氨基酸类酰胺化合物;
[0025]
(2)含n-boc-l-氨基酸类酰胺化合物经过脱保护和三氟乙酰化,得到含有n-三氟乙酰基氨基酸衍生物;
[0026]
所述脱保护在三氟乙酸的作用下进行;
[0027]
所述的三氟乙酰化所用的试剂为三氟乙酸酐。
[0028]
本发明还提供了一种所述的n-(2-苯甲酰基苯基)-2-三氟乙酰氨基烷酰胺衍生物的应用,包括:
[0029]
在碳酸铯的作用下,n-(2-苯甲酰基苯基)-2-三氟乙酰氨基烷酰胺衍生物在甲醇和水的混合溶剂中进行环合反应,得到所述的5-芳基-1,4-苯二氮杂-2-酮类化合物;
[0030]
反应温度为60~70℃,反应时间为10~15小时。
[0031]
反应式如下:
[0032][0033]
同现有技术相比,本发明的有益效果体现在:
[0034]
该制备方法可以方便地合成n-(2-苯甲酰基苯基)-2-三氟乙酰氨基烷酰胺衍生物,所采用的原料可以通过便宜易得的邻甲苯胺衍生物制备,来源广泛。
附图说明
[0035]
图1为实施例1得到的化合物1a的核磁图谱;
[0036]
图2为实施例1得到的化合物1m的核磁图谱;
[0037]
图3为实施例2得到的化合物3b的核磁图谱;
[0038]
图4为实施例2得到的化合物3m的核磁图谱;
[0039]
图5为实施例3得到的化合物4b的核磁图谱;
[0040]
图6为实施例3得到的化合物4f的核磁图谱。
具体实施方式
[0041]
实施例1
[0042]
底物1的合成步骤:
[0043]
(1)在100ml的三口烧瓶中加入邻甲苯胺衍生物(10mmol)、n,n'-二环己基碳二亚胺(dcc)(2.48g,12mmol)、4-(二甲氨基)吡啶(dmap)(0.317g,2.6mmol)和二氯甲烷(30ml),搅拌溶解。随后,在0℃下将含有n-boc-l-氨基酸衍生物(11mmol)的二氯甲烷(10ml)溶液滴加到上述混合液中。反应混合物在室温下搅拌大约12小时直到邻甲苯胺衍生物完全消耗。反应完成后,过滤沉淀的二环己基脲(dcu)。真空浓缩滤液,得到粗产物。
[0044]
(2)在冰水浴条件下,缓慢向上述粗品中滴加11ml三氟乙酸,滴加完毕后,继续搅拌大约1小时,在真空下去除多余的三氟乙酸,添加20ml二氯甲烷进行稀释,然后添加三乙胺直到溶液的ph值为微碱性。随后,在冰水浴条件下将含有4.0ml三氟乙酸酐的20ml二氯甲烷溶液缓慢滴加至上述系统并搅拌1小时。减压浓缩,在浓缩物中添加水(20ml)并搅拌。用乙酸乙酯(3
×
20ml)萃取该水溶液,合并有机相,用20ml水洗涤一次,并使用无水硫酸钠进行干燥。过滤,减压蒸馏。使用乙酸乙酯/石油醚作为洗脱液经硅胶柱层析分离纯化浓缩物,得到目标产物1(1a、1m、1n、1o、1p、1q、1r、1s、1t、1u、1v、1w、1x、1y或1z)。
[0045]
反应式如下:
[0046][0047]
得到的产物的结构和表征数据如下:
[0048][0049]
3-methyl-n-(o-tolyl)-2-(2,2,2-trifluoroacetamido)butanamide(1a,cas no.1808766-08-6).2.48g,82%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:10,v/v);mp 197
–
205℃;1h nmr(400mhz,dmso)δ9.68(s,1h),9.67(s,1h),7.33(d,j=7.6hz,1h),7.22(d,j=7.4hz,1h),7.17(t,j=7.1hz,1h),7.11(t,j=6.9hz,1h),4.38(t,j=8.6hz,1h),2.24
–
2.17(m,4h),1.01(d,j=6.8hz,3h),0.96(d,j=6.7hz,3h).
13
c nmr(100mhz,dmso)δ169.1,157.0(q,j=36.6hz),136.1,132.5,130.8,126.4,126.1,125.8,116.4(q,j=288.1hz),60.1,30.1,19.5,19.2,18.2.hrms(esi)calcd for c
14h17
f3n2o2[m+h]
+
303.1315,found 303.1324.
[0050][0051]
2,2,2-trifluoro-n-(2-oxo-2-(o-tolylamino)ethyl)acetamide(1m,cas no.357158-15-7).1.04g,40%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:5,v/v);mp 146
–
148℃;δ1h nmr(500mhz,dmso)δ9.78(s,1h),9.53(s,1h),7.40(d,j=7.8hz,1h),7.22(d,j=7.4hz,1h),7.17(t,j=7.5hz,1h),7.09(t,j=7.3hz,1h),4.07(d,j=5.6hz,2h),2.21(s,3h).
13
c nmr(125mhz,dmso)δ165.9,156.8(q,j=36.4hz),135.8,131.7,130.3,126.0,125.3,124.9,115.9(q,j=287.9hz),42.3,17.7.hrms(esi)calcd for c
11h11
f3n2o2[m+h]
+
261.0845,found 261.0846.
[0052][0053]
n-(o-tolyl)-2-(2,2,2-trifluoroacetamido)propanamide(1n,cas no.2210945-86-9).1.62g,59%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:5,v/v);mp 162
–
164℃;1h nmr(400mhz,dmso)δ9.72(s,1h),9.56(s,1h),7.32(d,j=7.6hz,1h),7.22(d,j=7.3hz,1h),7.17(t,j=7.3hz,1h),7.11(t,j=7.2hz,1h),4.57(q,j=7.0hz,1h),2.19(s,3h),1.45(d,j=7.1hz,3h).
13
c nmr(100mhz,dmso)δ171.1,156.7(q,j=36.5hz),136.2,132.7,130.8,126.4,126.0,125.8,116.3(q,j=287.9hz),49.9,18.1,17.9.hrms(esi)calcd for c
12h13
f3n2o2[m+h]
+
275.1002,found 275.1001.
[0054][0055]
(o-tolyl)-1-(2,2,2-trifluoroacetamido)cyclopropane-1-carboxamide(1p)
.1.94g,68%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:10,v/v);mp 208
–
211℃;1h nmr(400mhz,dmso)δ9.97(s,1h),9.46(s,1h),7.23(d,j=6.9hz,1h),7.18
–
7.13(m,3h),2.14(s,3h),1.44(dd,j=7.7,4.5hz,2h),1.07(dd,j=7.7,4.5hz,2h).
13
c nmr(100mhz,dmso)δ169.1,158.4(q,j=36.7hz),136.5,134.4,130.7,127.1,126.5,126.4,116.2(q,j=287.7hz),35.1,18.0,16.6.hrms(esi)calcd for c
13h13
f3n2o2[m+h]
+
287.1002,found 287.1009.
[0056][0057]
3-phenyl-n-(o-tolyl)-2-(2,2,2-trifluoroacetamido)propanamide(1r,cas no.2210438-10-9).2.73 g,78%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:10,v/v);mp 189
–
191℃;1h nmr(400mhz,dmso)δ9.87(d,j=8.1hz,1h),9.73(s,1h),7.37(d,j=7.1hz,2h),7.31(t,j=7.4hz,3h),7.25
–
7.16(m,3h),7.1(t,j=7.3hz,1h),4.88
–
4.82(m,1h),3.23(dd,j=13.6,5.0hz,1h),3.09(dd,j=13.5,10.3hz,1h),2.14(s,3h).
13
c nmr(100mhz,dmso)δ169.0,156.8(q,j=36.6hz),137.6,136.0,132.8,130.8,129.7,128.6,127.1,126.4,126.2,125.9,116.2(q,j=288.0hz),55.6,37.4,18.1.hrms(esi)calcd for c
18h17
f3n2o2[m+h]
+
351.1315,found 351.1315.
[0058][0059]
4-(methylthio)-n-(o-tolyl)-2-(2,2,2-trifluoroacetamido)butanamide(1s).1.37g,41%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:5,v/v);mp 150
–
153℃;1h nmr(400mhz,dmso)δ9.76(d,j=7.5hz,1h),9.65(s,1h),7.35
–
7.28(m,1h),7.22(d,j=7.4hz,1h),7.18(dd,j=10.6,4.4hz,1h),7.12(td,j=7.3,1.2hz,1h),4.69
–
4.58(m,1h),2.63
–
2.54(m,1h),2.53
–
2.49(m,1h),2.19(s,3h),2.16
–
2.10(m,2h),2.09(s,3h).
13
c nmr(100mhz,dmso)δ169.2,157.1(q,j=36.5hz),136.1,132.9,130.8,126.5,126.2,126.0,115.7(q,j=287.9hz),53.6,31.1,30.2,18.2,15.0.hrms(esi)calcd for c
14h17
f3n2o2s[m+h]
+
335.1036,found 335.1038.
[0060][0061]
3-(1h-indol-3-yl)-n-(o-tolyl)-2-(2,2,2-trifluoroacetamido)propanamide(1t).1.95g,49%yield;white solid after purification by column chromatography
(eluent,ethyl acetate/petroleum ether=1:5,v/v);mp 192
–
193℃;1nmr(400mhz,)δ10.88(s,1h),9.81(d,j=7.7hz,1h),9.74(s,1h),7.72(d,j=7.8hz,1h),7.35(d,j=8.0hz,1h),7.27(d,j=7.2,1h),7.25
–
7.18(m,2h),7.18
–
7.15(m,1h),7.12
–
7.06(m,2h),7.00(t,j=7.4hz,1h),4.96
–
4.68(m,1h),3.35
–
3.27(m,1h),3.21(dd,j=14.5,9.6hz,1h),2.12(s,3h).
13
nmr(100mhz,)δ169.5,156.8(q,j=42.4hz),136.5,136.1,133.0,130.8,127.5,126.4,126.2,126.1,124.4,121.5,119.0,118.8,116.3(q,j=288.0hz),111.8,109.9,55.1,27.7,18.1.hrms(esi)calcd for c
20h18
f3n3o2[m+h]
+
390.1424,found 390.1424.
[0062][0063]
n-(2,4-dimethylphenyl)-3-methyl-2-(2,2,2-trifluoroacetamido)butana mide(1u).2.34g,74%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:10,v/v);mp 219
–
221℃;1h nmr(400mhz,dmso)δ9.65(d,1h),9.59(s,1h),7.18(d,j=8.0hz,1h),7.03(s,1h),6.97(d,j=8.0hz,1h),4.36(t,j=8.8hz,1h),2.25(s,3h),2.23
–
2.17(m,1h),2.15(s,3h),1.00(d,j=6.7hz,3h),0.96(d,j=6.6hz,3h).
13
c nmr(100mhz,dmso)δ169.0,157.0(q,j=36.6),135.2,133.5,132.5,131.3,126.9,125.8,116.3(q,j=288.1hz),60.1,30.1,20.90,19.5,19.2,18.2.hrms(esi)calcd for c
15h19
f3n2o2[m+h]
+
317.1472,found 317.1472.
[0064][0065]
n-(4-methoxy-2-methylphenyl)-3-methyl-2-(2,2,2-trifluoroacetamido)butanamide(1v).2.09g,63%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:10,v/v);mp 219
–
221℃;1h nmr(400mhz,dmso)δ9.64(d,j=8.3hz,1h),9.56(s,1h),7.15(d,j=8.7hz,1h),6.80(d,j=2.8hz,1h),6.74(dd,j=8.7,2.9hz,1h),4.32(t,j=8.5hz,1h),3.72(s,3h),2.24
–
2.18(m,1h),2.16(s,3h),1.00(d,j=6.8hz,3h),0.95(d,j=6.7hz,3h).
13
c nmr(100mhz,dmso)δ169.1,157.5,157.0(q,j=36.6hz),134.6,129.0,127.4,116.4(q,j=286.3hz),115.8,111.7,60.0,55.6,30.1,19.5,19.2,18.4.hrms(esi)calcd for c
15h19
f3n2o3[m+h]
+
333.1421,found 333.1421.
[0066][0067]
n-(4-fluoro-2-methylphenyl)-3-methyl-2-(2,2,2-trifluoroacetamido)bu tanamide(1w).2.27g,71%yeild;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:10,v/v);mp 214
–
219℃;1h nmr(400mhz,dmso)δ9.71(s,1h),9.68(s,1h),7.30(dd,j=8.7,5.6hz,1h),7.10(dd,j=9.7,2.8hz,1h),7.01(td,j=8.6,2.9hz,1h),4.34(t,j=8.4hz,1h),2.25
–
2.16(m,4h),1.01(d,j=6.8hz,3h),0.96(d,j=6.7hz,3h).
13
c nmr(100mhz,dmso)δ169.2,160.1(d,j=241.8hz),157.1(q,j=36.8hz),135.6(d,j=8.3hz),132.3,127.8(d,j=8.7hz),117.1(d,j=22.2hz),116.4(q,j=286.3),113.0(d,j=22.2hz),60.1,30.0,19.5,19.2,18.2.hrms(esi)calcd for c
14h16
f4n2o2[m+h]
+
321.1221,found 321.1224.
[0068][0069]
n-(4-chloro-2-methylphenyl)-3-methyl-2-(2,2,2-trifluoroacetamido)bu tanamide(1x).2.42g,72%yeild;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:10,v/v);mp 230
–
232℃;1h nmr(400mhz,dmso)δ9.73(s,1h),9.70(d,j=8.0hz,1h),7.37(d,j=8.5hz,1h),7.32(d,j=2.1hz,1h),7.23(dd,j=8.5,2.3hz,1h),4.36(t,j=8.4hz,1h),2.22
–
2.17(m,4h),0.99(d,j=6.7hz,3h),0.95(d,j=6.7hz,3h).
13
c nmr(100mhz,dmso)δ169.3,157.1(q,j=36.7hz),135.1,134.9,130.4,129.9,127.3,126.3,116.4(q,j=288.1hz),60.1,30.0,19.5,19.2,18.0.hrms(esi)calcd for c
14h16
clf3n2o2[m+h]
+
337.0925,found 337.0926.
[0070][0071]
n-(2,3-dimethylphenyl)-3-methyl-2-(2,2,2-trifluoroacetamido)butana mide(1y).2.18g,69%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:10,v/v);mp 219
–
222℃;1h nmr(400mhz,dmso)δ9.72(s,1h),9.67(d,j=8.1hz,1h),7.11
–
7.07(m,1h),7.06
–
7.01(m,2h),4.37(t,j=8.4hz,1h),2.25(s,3h),2.22
–
2.19(m,1h),2.07(s,3h),1.01(d,j=6.8hz,3h),0.96(d,j=6.7hz,3h).
13
c nmr(100mhz,dmso)δ169.2,157.0(q,j=36.5hz),137.5,135.9,131.8,127.7,125.7,124.1,116.4(q,j=287.9hz),60.1,30.1,20.5,19.5,19.3,14.4.hrms(esi)calcd for c
15h19
f3n2o2[m+h]
+
317.1472,found 317.1472.
[0072][0073]
n-(2-benzylphenyl)-3-methyl-2-(2,2,2-trifluoroacetamido)butanamide(1z).2.57g,68%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:10,v/v);mp 227
–
228℃;1h nmr(400mhz,dmso)δ9.85(s,1h),9.69(s,1h),7.34(d,j=7.6hz,1h),7.24
–
7.19(m,3h),7.17
–
7.14(m,5h),4.41(s,1h),4.14
–
3.80(m,2h),2.15(dd,j=14.2,7.1hz,1h),0.92(dd,j=19.0,6.6hz,6h);
13
c nmr(100mhz,dmso)δ169.4,157.0(q,j=36.7hz),140.7,136.1,135.7,
130.6,129.2,128.7,126.9,126.6,126.4,126.4,116.4(d,j=288.2hz),59.9,36.8,30.3,19.4,19.1.hrms(esi)calcd for c
20h21
f3n2o2[m+h]
+
379.1628,found 379.1629.
[0074]
实施例2底物3的合成
[0075]
在空气下,将含有n-三氟乙酰基氨基酸衍生物1(0.25mmol)、芳基碘化物2(0.75mmol)、钯催化剂(0.05mmol,20mol%)、银盐(0.5mmol)、分子筛和溶剂(2.5ml)的混合物置于带有聚四氟乙烯帽的35ml压力管中。将反应管在130℃下加热24小时。反应结束后,将反应混合物冷却至室温,用乙酸乙酯(3ml)稀释,并通过硅藻土过滤。然后,向滤液中加入15ml水,并用乙酸乙酯(15ml)萃取混合物3次。合并有机相,用无水硫酸钠干燥,过滤,有机相真空浓缩。浓缩物经硅胶柱层析分离,乙酸乙酯/石油醚(ea/pe=1:7~1:20,v/v)洗脱,得到目标产物3
[0076][0077]
实施例3底物3的合成
[0078]
在空气下,将含有n-三氟乙酰基氨基酸衍生物1(0.25mmol)、芳基碘化物2(0.75mmol)、醋酸钯(0.05mmol,20mol%)、碳酸银(0.5mmol)、分子筛和dmac(2.5ml)的混合物置于带有聚四氟乙烯帽的35ml压力管中。将反应管在130℃下加热24小时。反应结束后,将反应混合物冷却至室温,用乙酸乙酯(3ml)稀释,并通过硅藻土过滤。然后,向滤液中加入15ml水,并用乙酸乙酯(15ml)萃取混合物3次。合并有机相,用无水硫酸钠干燥,过滤,
有机相真空浓缩。浓缩物经硅胶柱层析分离,乙酸乙酯/石油醚(ea/pe=1:7~1:20,v/v)洗脱,得到目标产物3(3a~3i)。
[0079]
化学式如下:
[0080][0081]
反应条件和反应结果如表2或表3所示:
[0082]
表2
[0083][0084]
表3
[0085]
[0086][0087]
部分化合物的表征数据如下:
[0088][0089]
n-(2-benzoylphenyl)-3-methyl-2-(2,2,2-trifluoroacetamido)butanamide(3a).85mg,87%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:20,v/v);mp159-162℃;1h nmr(400mhz,cdcl3)δ11.29(s,1h),8.62(d,j=8.2hz,1h),7.71(d,j=7.2hz,2h),7.64
–
7.60(m,3h),7.51(t,j=7.6hz,2h),7.19
–
7.12(m,2h),4.60(dd,j=8.3,5.2hz,1h),2.37
–
2.29(m,1h),1.08(dd,j=12.8,6.8hz,6h).
13
c nmr(100mhz,cdcl3)δ199.8,168.4,157.3(q,j=37.1hz),139.5,138.3,134.5,133.9,132.7,129.9,128.4,123.4,123.1,121.5,115.8(q,j=287.6hz),59.6,32.2,19.01,17.8.hrms(esi)calcd for c
20h19
f3n2o3[m+h]
+
393.1421,found 393.1425.
[0090][0091]
3-methyl-n-(2-(4-methylbenzoyl)phenyl)-2-(2,2,2-trifluoroacetamido)but anamide(3b).79mg,78%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:20,v/v);mp 105
–
108℃;1h nmr(400mhz,cdcl3)δ11.20(s,1h),8.58(d,j=8.0hz,1h),7.70
–
7.52(m,4h),7.31(d,j=7.9hz,2h),7.16(td,j=7.7,1.0hz,2h),4.59(dd,j=8.3,5.2hz,1h),2.46(s,3h),2.38
–
2.27(m,1h),1.04(dd,j=12.7,6.8hz,6h).
13
c nmr(100mhz,cdcl3)δ199.4,168.3,157.0(q,j=37.2hz),143.8,139.3,135.5,134.2,133.7,130.2,129.1,123.7,123.0,121.5,115.8(q,j=290.4hz),59.5,32.2,21.7,19.0,17.8.hrms(esi)calcd for c
21h21
f3n2o3[m+h]
+
407.1577,found 407.1584.
[0092][0093]
3-methyl-n-(2-(3-methylbenzoyl)phenyl)-2-(2,2,2-trifluoroacetamido)but anamide(3c).65mg,64%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:20,v/v);mp 108-110℃;1h nmr(400mhz,cdcl3)δ11.29(s,1h),8.60(d,j=8.3hz,1h),7.65
–
7.58(m,2h),7.52(s,1h),7.47(d,j=7.4hz,1h),7.43(d,j=7.6hz,1h),7.38(t,j=7.5hz,1h),7.29
–
7.26(m,1h),7.20
–
7.13(m,1h),4.60(dd,j=8.3,5.4hz,1h),2.43(s,3h),2.39
–
2.20(m,1h),1.04(dd,j=12.3,6.8hz,6h).
13
c nmr(100mhz,cdcl3)δ200.1,168.5,157.1(q,j=37.6hz),139.5,138.4,138.3,134.4,134.0,133.5,130.3,128.2,127.2,123.5,123.1,121.4,115.8(q,j=287.8hz),59.6,32.1,21.4,19.0,17.8.hrms(esi)calcd for c
21h21
f3n2o3[m+h]
+
407.1577,found 407.1581.
[0094][0095]
n-(2-(4-ethylbenzoyl)phenyl)-3-methyl-2-(2,2,2-trifluoroacetamido)butan amide(3e).76mg,72%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:20,v/v);mp 113
–
116℃;1h nmr(400mhz,cdcl3)δ11.21(s,1h),8.58(d,j=8.3hz,1h),7.71
–
7.54(m,4h),7.32(d,j=8.1hz,2h),7.24(s,1h),7.16(t,j=7.6hz,1h),4.59(dd,j=8.4,5.3hz,1h),2.75(q,j=7.6hz,2h),2.37
–
2.28(m,1h),1.29(t,j=7.6hz,3h),1.04(dd,j=12.7,6.8hz,6h).
13
c nmr(100mhz,cdcl3)δ199.4,168.4,157.1(q,j=37.5hz),149.9,139.3,135.7,134.2,133.7,130.3,127.9,123.8,123.0,121.5,130.8(q,j=287.8hz),59.6,32.1,29.0,19.0,17.8,15.2.hrms(esi)calcd for c
22h23
f3n2o3[m+h]
+
421.1734,found 421.1739.
[0096][0097]
n-(2-(4-(tert-butyl)benzoyl)phenyl)-3-methyl-2-(2,2,2-trifluoroacetamido)butanamide(3f).65mg,58%yield;yellow liquid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:20,v/v);1h nmr(400mhz,cdcl3)δ11.24(s,1h),8.59(d,j=8.2hz,1h),7.71
–
7.64(m,3h),7.60(t,j=7.9hz,1h),7.52(s,1h),7.50(s,1h),7.27(d,j=6.4hz,1h),7.16(t,j=7.6hz,1h),4.59(dd,j=8.3,5.3hz,1h),2.37
–
2.28(m,1h),1.37(s,9h),1.04(dd,j=
chromatography(eluent,ethyl acetate/petroleum ether=1:15,v/v);mp 121
–
122℃;1h nmr(400mhz,cdcl3)δ11.00(s,1h),8.54(d,j=8.0hz,1h),7.78
–
7.69(m,2h),7.66
–
7.52(m,2h),7.21
–
7.10(m,2h),7.06
–
6.92(m,2h),4.57(dd,j=8.3,5.2hz,1h),3.91(s,3h),2.36
–
2.26(m,1h),1.04(dd,j=12.0,6.8hz,6h).
13
c nmr(100mhz,cdcl3)δ197.9,168.2,163.6,157.1(q,j=37.2hz),138.9,133.8,133.2,132.7,130.5,129.2,124.3,123.1,121.6,115.8(d,j=287.2hz),113.7,59.5,55.6,32.2,19.0,17.8.hrms(esi)calcd for c
22h21
f3n2o5[m+h]
+
451.1476,found 451.1480.
[0104][0105]
4-(2-(3-methyl-2-(2,2,2-trifluoroacetamido)butanamido)benzoyl)benzoate(3j).84mg,72%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:15,v/v);mp 129
–
131℃;1h nmr(400mhz,cdcl3)δ11.33(s,1h),8.65(d,j=8.4hz,1h),8.17(d,j=8.2hz,2h),7.74(d,j=8.2hz,2h),7.64(t,j=7.9hz,1h),7.57(d,j=7.8hz,1h),7.32
–
7.22(m,1h),7.17(t,j=7.6hz,1h),4.61(dd,j=8.2,5.6hz,1h),4.44(q,j=7.1hz,2h),2.47
–
2.16(m,1h),1.43(t,j=7.1hz,3h),1.06(dd,j=11.6,6.8hz,6h).
13
c nmr(100mhz,cdcl3)δ199.3,168.6,165.6,157.1(q,j=37.7hz),141.9,139.8,135.1,134.0,133.8,129.5,129.5,123.2,122.8 121.5,115.8(q,j=287.8hz),61.5,59.7,32.0,19.0,17.8,14.3.hrms(esi)calcd for c
23h23
f3n2o5[m+h]
+
465.1632,found 465.1638.
[0106][0107]
nn-(2-(4-cyanobenzoyl)phenyl)-3-methyl-2-(2,2,2-trifluoroacetamido)buta namide(3k).73mg,70%yield;yellow liquid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:15,v/v);1h nmr(400mhz,cdcl3)δ11.24(s,1h),8.66(d,j=8.0hz,1h),7.80(q,j=8.5hz,4h),7.67(t,j=7.9hz,1h),7.53(dd,j=7.9,1.4hz,1h),7.23
–
7.11(m,2h),4.59(dd,j=8.3,5.4hz,1h),2.38
–
2.29(m,1h),1.06(dd,j=12.1,6.8hz,6h).
13
c nmr(100mhz,cdcl3)δ198.2,168.6,157.1(q,j=37.3hz),141.9,140.0,135.5,133.7,132.2,130.1,123.3,122.2,121.7,115.8(q,j=279.4hz),117.8,115.9,59.7,32.0,19.0,17.8.hrms(esi)calcd for c
21h18
f3n3o3[m+h]
+
418.1373,found 418.1369.
[0108][0109]
3-methyl-n-(2-(4-nitrobenzoyl)phenyl)-2-(2,2,2-trifluoroacetamido)butan amide(3l).71mg,65%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:15,v/v);mp 164
–
167℃;1h nmr(400mhz,cdcl3)δ11.28(s,1h),8.68(d,j=7.8hz,1h),8.42
–
8.30(m,2h),7.91
–
7.79(m,2h),7.74
–
7.63(m,1h),7.53(dd,j=7.9,1.4hz,1h),7.23
–
7.16(m,1h),7.12(d,j=8.1hz,1h),4.60(dd,j=8.3,5.4hz,1h),2.39
–
2.30(m,1h),1.06(dd,j=12.1,6.8hz,6h).
13
c nmr(100mhz,cdcl3)δ198.1,168.6,158.7(q,j=25.2hz),149.9,143.6,140.1,135.7,133.8,130.5,123.7,123.3,122.1,121.7,115.9(q,j=267.5hz),59.7,32.0,19.0,17.8.hrms(esi)calcd for c
20h18
f3n3o5[m+h]
+
438.1272,found 438.1272.
[0110][0111]
n-(2-((2-benzoylphenyl)amino)-2-oxoethyl)-2,2,2-trifluoroacetamide(3m).61mg,70%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:7,v/v);mp 112
–
114℃;1h nmr(500mhz,cdcl3)δ11.22(s,1h),8.55(d,j=8.6hz,1h),7.69(d,j=7.6hz,2h),7.65
–
7.56(m,3h),7.49(t,j=7.7hz,2h),7.40(s,1h),7.16(t,j=7.6hz,1h),4.24(d,j=4.9hz,2h).
13
c nmr(100mhz,cdcl3)δ199.7,165.5,157.3(q,j=37.8hz),139.4,138.2,134.4,133.8,132.7,129.9,128.4,123.4,123.1,121.6,115.7(q,j=287.5hz),43.6.hrms(esi)calcd for c
17h13
f3n2o3[m+h]
+
351.0951,found 351.0959.
[0112]
实施例4底物4的合成步骤:
[0113][0114]
在带有聚四氟乙烯盖的35ml压力管中,加入化合物3(0.11mmol)、碳酸铯(0.072g,0.22mmol)和2ml甲醇/水(1:1)。试管在65℃下加热12小时。将反应混合物冷却至室温,用乙酸乙酯(3ml)稀释,通过硅藻土过滤,滤液减压浓缩。浓缩物用乙酸乙酯/石油醚通过硅胶柱层析分离纯化,得到产物4(ea/pe=1:2~1:5)。
[0115]
反应式和反应产物如下:
[0116][0117]
3-isopropyl-5-phenyl-1,3-dihydro-2h-benzo[e][1,4]diazepin-2-one(4a,cas no.34124-69-1).25mg,82%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:5,v/v);mp 200
–
202℃;1h nmr(400mhz,cdcl3)δ8.88(s,1h),7.62
–
7.50(m,3h),7.51
–
7.43(m,1h),7.43
–
7.35(m,3h),7.19(t,j=7.5hz,2h),3.16(d,j=9.2hz,1h),2.81
–
2.71(m,1h),1.24(d,j=6.7hz,3h),1.11(dd,j=6.5,3.3hz,3h).
13
c nmr(100mhz,cdcl3)δ170.9,168.7,139.5,138.4,131.5,131.1,130.2,129.8,128.2,127.6,123.2,121.1,69.3,29.0,20.4,19.1.hrms(esi)calcd for c
18h18
n2o[m+h]
+
279.1492,found 279.1489.
[0118][0119]
3-isopropyl-5-(p-tolyl)-1,3-dihydro-2h-benzo[e][1,4]diazepin-2-one(4b).30mg,93%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:5,v/v);mp 214
–
215℃;1h nmr(400mhz,cdcl3)δ9.02(s,1h),7.57
–
7.48(m,1h),7.46(d,j=7.7hz,2h),7.38(d,j=7.7hz,1h),7.18
–
7.12(m,4h),3.12(d,j=9.3hz,1h),2.77(dd,j=15.5,6.6hz,1h),2.39(s,3h),1.23(d,j=6.7hz,3h),1.09(dd,j=6.5,1.3hz,3h).
13
c nmr(100mhz,cdcl3)δ171.1,168.5,140.4,138.4,136.8,131.4,131.1,129.8,128.8,127.8,123.1,121.1,69.2,29.0,21.4,20.4,19.1.hrms(esi)calcd for c
19h20
n2o[m+h]
+
293.1649,found 293.1646.
[0120][0121]
3-isopropyl-5-(4-methoxyphenyl)-1,3-dihydro-2h-benzo[e][1,4]diazep in-2-one(4c,cas no.152236-83-4).32mg,94%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether =1:5,v/v);mp 227
–
228℃;1h nmr(400mhz,cdcl3)δ9.48(s,1h),7.53
–
7.49(m,3h),7.36(d,j=7.2hz,1h),7.19(d,j=8.1hz,1h),7.14(t,j=7.6hz,1h),6.86(d,j=8.8hz,2h),3.82(s,3h),3.08(d,j=9.2hz,1h),2.83
–
2.57(m,1h),1.20(d,j=6.7hz,3h),1.09(d,j=6.5hz,3h).
13
c nmr(100mhz,cdcl3)δ171.5 168.0,161.3,138.5,132.1,131.4,131.4,131.1,127.8,123.1,121.3,111.5,69.2,55.4,29.0,20.4,19.1.hrms(esi)calcd for c
19h20
n2o2[m+h]
+
309.1598,found 309.1621.
[0122][0123]
4-(3-isopropyl-2-oxo-2,3-dihydro-1h-benzo[e][1,4]diazepin-5-yl)benzoate(4d).32mg,86%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:3,v/v);mp 230
–
232℃;1h nmr(400mhz,cdcl3)δ9.65(d,j=40.0hz,1h),8.07
–
7.97(m,2h),7.67
–
7.46(m,2h),7.52(t,j=7.7hz,1h),7.32
–
7.19(m,2h),7.15(t,j=7.1hz,1h),3.92(s,3h),3.15(d,j=9.1hz,1h),2.87
–
2.7(m,1h),1.22(d,j=6.6hz,3h),1.09(dd,j=17.5,6.5hz,3h).
13
c nmr(100mhz,cdcl3)δ171.0,168.0,167.0,143.4,138.6,131.8,131.4,130.7,129.8,129.4,127.1,123.3,121.4,69.6,52.3,29.0,20.4,19.1.hrms(esi)calcd for c
20h20
n2o3[m+h]
+
337.1547,found 337.1544.
[0124][0125]
3-isopropyl-7-methyl-5-phenyl-1,3-dihydro-2h-benzo[e][1,4]diazepin-2-one(4e).29 mg,89%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:5,v/v);mp 212
–
214℃;1h nmr(400mhz,cdcl3)δ8.37(s,1h),7.65
–
7.51(m,2h),7.46
–
7.41(m,1h),7.41
–
7.34(m,2h),7.32(dd,j=8.2,1.5hz,1h),7.13(s,1h),7.04(d,j=8.2hz,1h),3.11(d,j=9.2hz,1h),
2.93
–
2.67(m,1h),2.32(s,3h),1.20(d,j=6.7hz,3h),1.07(dd,j=6.4,4.2hz,3h).
13
c nmr(100mhz,cdcl3)δ170.7,168.6,139.6,136.0,133.1,132.5,131.0,130.1,129.8,128.2,127.6,120.9,69.3,29.0,20.8,20.3,19.1.hrms(esi)calcd for c
19h20
n2o[m+h]
+
293.1649,found 293.1646.
[0126][0127]
3-isopropyl-7-methoxy-5-phenyl-1,3-dihydro-2h-benzo[e][1,4]diazepin-2-one(4f,cas no.2226130-96-5).31mg,91%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:5,v/v);mp 191
–
192℃;1h nmr(400mhz,cdcl3)δ9.50(d,j=25.4hz,1h),7.65
–
7.52(m,2h),7.46
–
7.29(m,3h),7.15(t,j=7.3hz,1h),7.07(dd,j=8.9,2.8hz,1h),6.81
–
6.78(m,1h),3.71(d,j=2.3hz,3h),3.12(d,j=9.3hz,1h),2.81
–
2.71(m,1h),1.21(d,j=6.7hz,3h),1.08(dd,j=18.7,6.5hz,3h).
13
c nmr(100 mhz,cdcl3)δ171.0,168.2,154.9,139.3,132.3,130.2,129.8,128.5,128.2,122.7,118.7,114.2,69.4,55.7,29.0,20.4,19.2.hrms(esi)calcd for c
19h20
n2o2[m+h]
+
309.1598,found 309.1585.
[0128][0129]
7-chloro-3-isopropyl-5-phenyl-1,3-dihydro-2h-benzo[e][1,4]diazepin-2-one(4g,cas no.14404-96-7).33mg,97%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:5,v/v);mp 225
–
226℃;1h nmr(400mhz,cdcl3)δ10.01(d,j=3.9hz,1h),7.57
–
7.49(m,2h),7.44(m,2h),7.41
–
7.34(m,2h),7.31(d,j=1.6hz,1h),7.17(d,j=8.7hz,1h),3.10(d,j=9.2hz,1h),2.88
–
2.60(m,1h),1.21(d,j=6.7hz,3h),1.08(dd,j=6.5,3.6hz,3h).
13
c nmr(100mhz,cdcl3)δ171.3,167.6,138.8,137.3,131.7,130.5,130.3,129.8,128.8,128.5,128.4,122.9,69.5,29.0,20.4,19.1.hrms(esi)calcd for c
18h17
cln2o[m+h]
+
313.1102,found 313.1111.
[0130][0131]
5-phenyl-1,3-dihydro-2h-benzo[e][1,4]diazepin-2-one(4h,cas no.2898-08-0).24mg,94%yield;white solid after purification by column chromatography(eluent,ethyl acetate/petroleum ether=1:2,v/v);mp 175
–
177℃;1h nmr(400mhz,
cdcl3)δ9.47(s,1h),7.51(m,3h),7.44(t,j=7.3hz,1h),7.38(t,j=7.5hz,2h),7.32(dd,j=7.8,1.0hz,1h),7.20(d,j=7.9hz,1h),7.18
–
7.11(m,1h),4.33(s,2h).
13
c nmr(100mhz,cdcl3)δ172.2,171.1,139.4,138.8,131.7,131.4,130.3,129.7,128.2,127.3,123.4,121.1,56.7.hrms(esi)calcd for c
15h12
n2o[m+h]
+
237.1023,found 237.1028。
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1