一种含烷基和芳基苯并喹啉类化合物的合成方法
二氯乙烷。
7.进一步优选,所述芳香醛类化合物1、β-萘胺类化合物2、三级脂肪胺类化合物3、碘试剂与氧化剂的投料摩尔比为1:1:3:1.5:2,芳香醛类化合物1与溶剂的投料配比为1mmol:4ml。
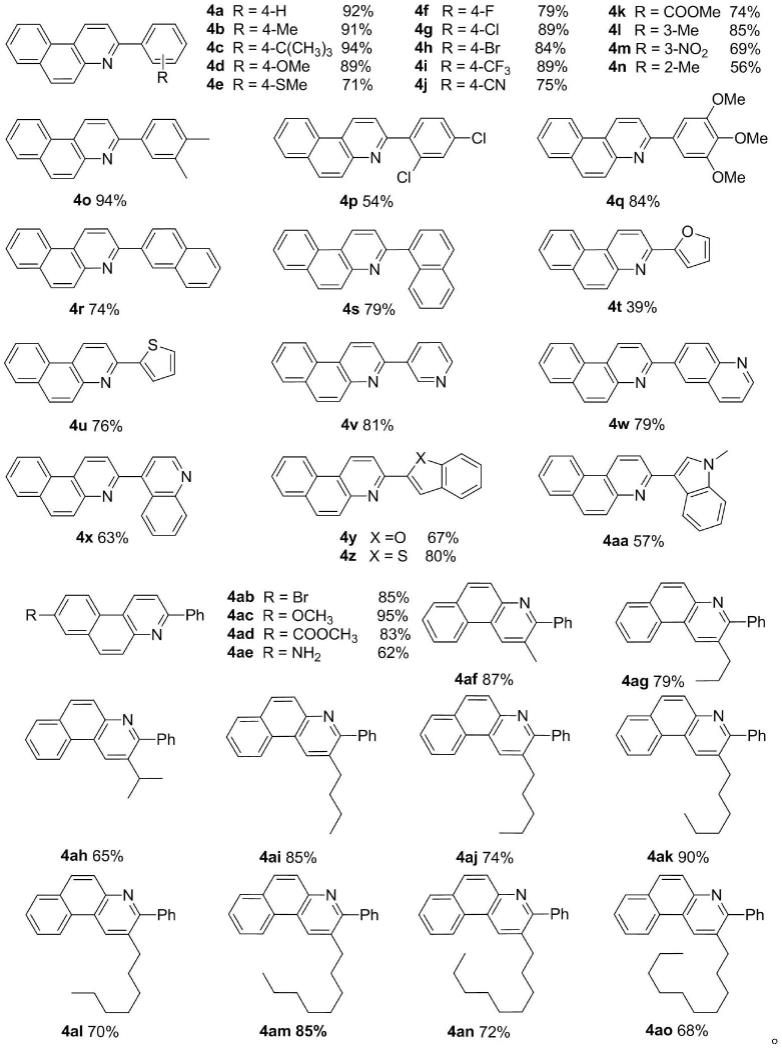
8.进一步优选,合成的目标产物含烷基和芳基苯并喹啉类化合物及对应的收率为:
[0009][0010]
本发明与现有技术相比具有以下优点:1、合成过程为无过渡金属催化的一锅串联反应,过程简单、高效;2、原料催化剂廉价易得,反应条件温和,操作简便,适合商业化、规模化工业生产;3、底物的适用范围广;4、以三级脂肪胺类化合物为原料能够在目标产物中巧妙地引入烷基取代基。因此,本发明为含烷基和芳基苯并喹啉类化合物的合成提供了一种经济实用且简单高效的新方法。
具体实施方式
[0011]
以下通过实施例对本发明的上述内容做进一步详细说明,但不应该将此理解为本发明上述主题的范围仅限于以下的实施例,凡基于本发明上述内容实现的技术均属于本发明的范围。
[0012]
实施例1
[0013][0014]
在35ml密封管中加入苯甲醛1a(53mg,0.5mmol)、β-萘胺2a(71.5mg,0.5mmol)、三乙胺3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4a(117mg,92%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.93(d,j=8.8hz,1h),8.57(d,j=8.0hz,1h),8.23
–
8.16(m,2h),8.07(d,j=9.2hz,1h),8.00
–
7.94(m,2h),7.93
–
7.89(m,1h),7.69
–
7.59(m,2h),7.57
–
7.50(m,2h),7.49
–
7.43(m,1h);
13
c nmr(100mhz,cdcl3):δ(ppm)156.8,148.1,139.4,131.6,131.4,130.9,129.6,129.2,128.8,128.7,128.6,127.4,127.1,127.0,124.1,122.6,118.7;hrms(esi):m/z[m+h]
+
calcd for c
19h14
n:256.1121;found:256.1123。
[0015]
实施例2
[0016]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、单质碘(190.5mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到目标产物4a(52.3mg,41%)。
[0017]
实施例3
[0018]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、过氧化二异丙苯(270mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到目标产物4a(99.5mg,78%)。
[0019]
实施例4
[0020]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、过硫酸钾(270mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到目标产物4a(57.4mg,45%)。
[0021]
实施例5
[0022]
在35ml反应瓶中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,
1.5mmol)、碘化铵(108.7mg,0.75mmol)和氯苯(2ml),然后置于120℃金属浴中敞口搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到目标产物4a(75mg,59%)。
[0023]
实施例6
[0024]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和甲苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到目标产物4a(111mg,87%)。
[0025]
实施例7
[0026]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和1,4-二氧六环(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到目标产物4a(100.7mg,79%)。
[0027]
实施例8
[0028]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和乙腈(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到目标产物4a(53.5mg,42%)。
[0029]
实施例9
[0030]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和n-甲基-2-吡咯烷酮(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到目标产物4a(62.5mg,49%)。
[0031]
实施例10
[0032]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和二甲亚砜(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到目标产物4a(68.5mg,54%)。
[0033]
实施例11
[0034]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和1,2-二氯乙烷(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),
之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到目标产物4a(107mg,84%)。
[0035]
实施例12
[0036]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和1,2-二氯乙烷(2ml),然后置于110℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到目标产物4a(104.5mg,82%)。
[0037]
实施例13
[0038]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和1,2-二氯乙烷(2ml),然后置于130℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到目标产物4a(117mg,92%)。
[0039]
实施例14
[0040][0041]
在35ml密封管中加入1b(60mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4b(122mg,91%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.91(d,j=8.4hz,1h),8.57(d,j=8.0hz,1h),8.12
–
8.08(m,2h),8.06(d,j=8.8hz,1h),7.99
–
7.94(m,2h),7.92(dd,j=7.6,1.2hz,1h),7.69
–
7.59(m,2h),7.33(d,j=8.0hz,2h),2.43(s,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)156.8,148.1,139.3,136.6,131.5,131.3,130.8,129.6(2),129.5(6),128.6(4),128.5(9),127.2,126.9(7),126.9(5),123.9,122.5,118.5,21.3;hrms(esi):m/z[m+h]
+
calcd for c
20h16
n:270.1277;found:270.1279。
[0042]
实施例15
[0043][0044]
在35ml密封管中加入1c(81mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4c(146mg,94%)。该化合物的
表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.96(d,j=8.8hz,1h),8.61(d,j=8.0hz,1h),8.15
–
8.11(m,2h),8.07(d,j=8.8hz,1h),8.01
–
7.97(m,2h),7.93(dd,j=8.0,1.2hz,1h),7.71
–
7.60(m,2h),7.58
–
7.54(m,2h),1.39(s,9h);
13
c nmr(100mhz,cdcl3):δ(ppm)156.8,152.4,148.2,136.6,131.5,131.3,130.8,129.6,128.6(4),128.6(2),127.1,127.0,126.9,125.8,123.9,122.5,118.7,34.7,31.3;hrms(esi):m/z[m+h]
+
calcd for c
23h22
n:312.1747;found:312.1748。
[0045]
实施例16
[0046][0047]
在35ml密封管中加入1d(68mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4d(127mg,89%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.92(d,j=8.8hz,1h),8.58(d,j=8.4hz,1h),8.19
–
8.15(m,2h),8.05(d,j=9.2hz,1h),7.98(d,j=9.2hz,1h),7.95
–
7.91(m,2h),7.70
–
7.60(m,2h),7.07
–
7.03(m,2h),3.88(s,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)160.7,156.4,148.1,131.9,131.5,131.4,130.8,129.7,128.6(9),128.6(6),128.5,127.0,126.9,123.6,122.5,118.2,114.2,55.4.hrms(esi):m/z[m+h]
+
calcd for c
20h16
no:286.1226;found:286.1226。
[0048]
实施例17
[0049][0050]
在35ml密封管中加入1e(76mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4e(107mg,71%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.96(d,j=8.8hz,1h),8.60(d,j=8.0hz,1h),8.17
–
8.13(m,2h),8.05(d,j=9.2hz,1h),7.98(dd,j=8.8,7.2hz,2h),7.94(dd,j=8.0,1.2hz,1h),7.71
–
7.61(m,2h),7.42
–
7.37(m,2h),2.55(s,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)156.1,148.2,140.2,136.0,131.6,131.5,131.0,129.6,128.7,128.5,127.6,127.0,126.4,124.0,122.6,118.3,15.5;hrms(esi):m/z[m+h]
+
calcd for c
20h16
ns:302.0998;found:302.0998。
[0051]
实施例18
[0052][0053]
在35ml密封管中加入1f(62mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4f(108mg,79%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.96(d,j=8.8hz,1h),8.60(d,j=8.0hz,1h),8.22
–
8.16(m,2h),8.02(q,j=9.2hz,2h),7.96
–
7.92(m,2h),7.72
–
7.62(m,2h),7.24
–
7.18(m,2h);
13
c nmr(100mhz,cdcl3):δ(ppm)165.0,162.5,155.7,148.1,135.6,135.5,131.6(1),131.5(8),131.1,129.5,129.3,129.2,128.7,128.4,127.1(4),127.1(1),124.0,122.6,118.4,115.9,115.7;hrms(esi):m/z[m+h]
+
calcd for c
19h13
fn:274.1027;found:274.1026。
[0054]
实施例19
[0055][0056]
在35ml密封管中加入1g(70.3mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4g(129mg,89%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.94(d,j=8.8hz,1h),8.58(d,j=8.0hz,1h),8.17
–
8.11(m,2h),8.01(q,j=9.2hz,2h),7.93(d,j=8.8hz,2h),7.71
–
7.62(m,2h),7.52
–
7.46(m,2h);
13
c nmr(100mhz,cdcl3):δ(ppm)155.4,148.2,137.8,135.4,131.7,131.6,131.2,129.5,129.0,128.7,128.6,128.4,127.2,127.1,124.2,122.6,118.3;hrms(esi):m/z[m+h]
+
calcd for c
19h13
cln:290.0731;found:290.0730。
[0057]
实施例20
[0058][0059]
在35ml密封管中加入1h(92.5mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4h(140mg,84%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.98(d,j=8.8hz,1h),8.61(d,j=8.0hz,
1h),8.11
–
8.07(m,2h),8.02(q,j=8.8hz,2h),7.98
–
7.93(m,2h),7.73
–
7.68(m,1h),7.68
–
7.63(m,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)155.5,148.2,138.2,132.0,131.6(8),131.6(5),131.2,129.5,128.9,128.7,128.4,127.3,127.2,124.3,123.8,122.6,118.3;hrms(esi):m/z[m+h]
+
calcd for c
19h13
brn:334.0226;found:334.0226。
[0060]
实施例21
[0061][0062]
在35ml密封管中加入1i(87mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4i(143.7mg,89%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.98(d,j=8.4hz,1h),8.60(d,j=8.0hz,1h),8.30(d,j=8.0hz,2h),8.07
–
7.97(m,3h),7.96
–
7.92(m,1h),7.78(d,j=8.4hz,2h),7.73
–
7.63(m,2h);
13
c nmr(100mhz,cdcl3):δ(ppm)155.1,148.2,142.6,131.8,131.7,131.4,131.1,130.8,129.4,128.8,128.4,127.6,127.4,127.2,125.7(9),125.7(6),125.7(2),125.6(8),125.5,124.6,122.8,122.7,118.7;hrms(esi):m/z[m+h]
+
calcd for c
20h13
f3n:324.0995;found:324.0994。
[0063]
实施例22
[0064][0065]
在35ml密封管中加入1j(65.5mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4j(105mg,75%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)9.03(d,j=8.8hz,1h),8.63(d,j=8.0hz,1h),8.35
–
8.30(m,2h),8.08
–
8.00(m,3h),7.96(dd,j=7.6,1.6hz,1h),7.84
–
7.79(m,2h),7.75
–
7.66(m,2h);
13
c nmr(100mhz,cdcl3):δ(ppm)154.3,148.2,143.4,132.6,131.9,131.8,131.6,129.3,128.8,128.3,127.9,127.6,127.3,124.8,122.8,118.9,118.6,112.6;hrms(esi):m/z[m+h]
+
calcd for c
20h13
n2:281.1073;found:281.1073。
[0066]
实施例23
[0067][0068]
在35ml密封管中加入1k(82mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,
1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=30/1)得到黄色固体产物4k(116mg,74%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.99(d,j=8.4hz,1h),8.62(d,j=8.0hz,1h),8.30
–
8.26(m,2h),8.21
–
8.18(m,2h),8.08
–
8.00(m,3h),7.94(dd,j=8.0,1.2hz,1h),7.73
–
7.63(m,2h),3.96(s,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)166.9,155.4,148.2,143.5,131.8,131.6,131.3,130.5,130.1,129.4,128.7,128.5,127.4,127.3,127.2,124.6,122.7,118.9,52.2;hrms(esi):m/z[m+h]
+
calcd for c
21h16
no2:314.1176;found:314.1174。
[0069]
实施例24
[0070][0071]
在35ml密封管中加入1l(60mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4l(114mg,85%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.96(d,j=8.8hz,1h),8.60(d,j=8.0hz,1h),8.10
–
8.04(m,2h),8.01
–
7.91(m,4h),7.71
–
7.61(m,2h),7.42(t,j=7.6hz,1h),7.28(d,j=7.6hz,1h),2.49(s,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)157.0,148.1,139.3,138.5,131.6,131.4,130.9,130.0,129.6,128.7(3),128.6(7),128.6,128.1,127.0(4),127.0(2),124.5,124.1,122.6,118.9,21.6;hrms(esi):m/z[m+h]
+
calcd for c
20h16
n:270.1277;found:270.1278。
[0072]
实施例25
[0073][0074]
在35ml密封管中加入1m(75.5mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=50/1)得到黄色固体产物4m(104mg,69%)。该化合物的表征数据如下:1hnmr(400mhz,cdcl3):δ(ppm)9.06(t,j=2.0hz,1h),9.01(d,j=8.4hz,1h),8.61(d,j=8.0hz,1h),8.58
–
8.53(m,1h),8.31
–
8.26(m,1h),8.07
–
8.00(m,3h),7.95(dd,j=8.0,1.2hz,1h),7.74
–
7.65(m,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)153.8,148.8,148.2,141.0,133.1,131.9,131.8,131.6,129.7,129.3,128.8,128.3,127.6,127.3,
124.8,123.7,122.8,122.2,118.2;hrms(esi):m/z[m+h]
+
calcd for c
19h13
n2o2:301.0972;found:301.0973。
[0075]
实施例26
[0076][0077]
在35ml密封管中加入1n(60mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4n(75mg,56%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)9.01(d,j=8.4hz,1h),8.67(d,j=8.0hz,1h),8.04(q,j=9.2hz,2h),7.96(dd,j=8.0,1.2hz,1h),7.75
–
7.69(m,2h),7.69
–
7.64(m,1h),7.58
–
7.54(m,1h),7.37
–
7.32(m,3h),2.46(s,3h);
13
c nmr(100mhz,cdcl3)δ(ppm):159.7,147.8,140.5,136.1,131.7,130.9(4),130.8(8),130.8,129.8,129.6,128.7,128.5,128.4,127.2,127.1,126.0,123.7,122.6,122.3,20.4;hrms(esi):m/z[m+h]
+
calcd for c
20h16
n:270.1277;found:270.1275。
[0078]
实施例27
[0079][0080]
在35ml密封管中加入1o(67mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4o(133mg,94%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.88(d,j=8.8hz,1h),8.55(d,j=8.0hz,1h),8.06(d,j=8.8hz,1h),8.01(s,1h),7.98
–
7.92(m,2h),7.91
–
7.87(mi,2h),7.66
–
7.57(m,2h),7.27(d,j=8.0hz,1h),2.38(s,3h),2.33(s,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)156.9,148.1,138.0,137.0,136.9,131.5,131.3,130.8,130.1,129.6,128.6(1),128.5(8),128.5,126.9(2),126.8(8),124.7,123.8,122.5,118.6,20.0,19.7;hrms(esi):m/z[m+h]
+
calcd for c
21h18
n:284.1434;found:284.1436。
[0081]
实施例28
[0082][0083]
在35ml密封管中加入1p(87.5mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,
1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4p(87.5mg,54%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)9.02(d,j=8.4hz,1h),8.66(d,j=7.6hz,1h),8.06
–
8.01(m,2h),7.97(dd,j=8.0,1.2hz,1h),7.92(d,j=8.4hz,1h),7.76
–
7.71(m,2h),7.70
–
7.66(m,1h),7.55(d,j=2.0hz,1h),7.42(dd,j=8.0,2.0hz,1h);
13
c nmr(100mhz,cdcl3):δ(ppm)155.5,148.1,137.9,135.1,133.1,132.7,131.8,131.3,130.6,129.9,129.4,128.8,128.2,127.5(4),127.4(8),127.3,124.4,122.8,122.6;hrms(esi):m/z[m+h]
+
calcd for c
19h12cl2
n:324.0341;found:324.0341。
[0084]
实施例29
[0085][0086]
在35ml密封管中加入1q(98mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=12/1)得到黄色固体产物4q(145mg,84%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.99(d,j=8.8hz,1h),8.63(d,j=8.4hz,1h),8.08(d,j=8.8hz,1h),8.01(d,j=9.2hz,1h),7.97(d,j=8.8hz,1h),7.95(dd,j=8.0,1.2hz,1h),7.73
–
7.63(m,2h),7.45(s,2h),4.03(s,6h),3.94(s,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)156.4,153.6,148.1,139.3,135.1,131.6,131.5,131.0,129.6,128.7,128.5,127.1(1),127.0(9),124.0,122.6,118.6,104.6,61.0,56.3;hrms(esi):m/z[m+h]
+
calcd for c
22h20
no3:346.1438;found:346.1437。
[0087]
实施例30
[0088][0089]
在35ml密封管中加入1r(78mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=50/1)得到黄色固体产物4r(113mg,74%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)9.03(d,j=8.4hz,1h),8.68(d,j=1.2hz,1h),8.65(d,j=8.4hz,1h),8.40(dd,j=8.4,2.0hz,1h),8.17(d,j=8.4hz,1h),8.13(d,j=9.2hz,1h),8.04
–
7.99(m,3h),7.96(dd,j=7.6,1.2hz,1h),7.92
–
7.88(m,1h),7.73
–
7.63(m,2h),7.56
–
7.51(m,2h);
13
c nmr(100mhz,cdcl3):δ(ppm)156.7,148.3,136.7,
133.8,133.5,131.7,131.5,131.1,129.6,128.8,128.7,128.6(0),128.5(8),127.7,127.1(4),127.0(9),126.9,126.7,126.3,124.9,124.2,122.7,119.0;hrms(esi):m/z[m+h]
+
calcd for c
23h16
n:306.1277;found:306.1278。
[0090]
实施例31
[0091][0092]
在35ml密封管中加入1s(78mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=50/1)得到黄色固体产物4s(120.5mg,79%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)9.05(d,j=8.4hz,1h),8.68(d,j=8.0hz,1h),8.19(d,j=8.0hz,1h),8.12(d,j=8.8hz,1h),8.04(d,j=8.8hz,1h),7.99
–
7.92(m,3h),7.85(d,j=8.8hz,1h),7.77
–
7.70(m,2h),7.69
–
7.65(m,1h),7.61(dd,j=8.0,7.2hz,1h),7.54
–
7.45(m,2h);
13
c nmr(100mhz,cdcl3):δ(ppm)158.9,148.0,138.5,134.0,131.7,131.3,131.1,131.0,129.6,129.1,128.7,128.5,128.4,127.8,127.2(4),127.1(5),126.6,125.9,125.7,125.4,124.0,123.2,122.7;hrms(esi):m/z[m+h]
+
calcd for c
23h16
n:306.1277;found:306.1275。
[0093]
实施例32
[0094][0095]
在35ml密封管中加入1t(48mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4t(48mg,39%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.97(d,j=8.8hz,1h),8.61(d,j=8.0hz,1h),8.06
–
7.97(m,3h),7.94(dd,j=7.6,1.2hz,1h),7.72
–
7.68(m,1h),7.67
–
7.62(m,2h),7.25(s,1h),6.61(dd,j=3.6,2.0hz,1h);
13
c nmr(100mhz,cdcl3):δ(ppm)153.6,148.7,148.1,144.0,131.6,131.4,131.2,129.6,128.7,128.2,127.1(3),127.1(0),124.0,122.5,117.3,112.2,109.8;hrms(esi):m/z[m+h]
+
calcd for c
17h12
no:246.0913;found:246.0913。
[0096]
实施例33
[0097][0098]
在35ml密封管中加入1u(56mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4u(99mg,76%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.88(d,j=8.4hz,1h),8.56(d,j=8.4hz,1h),8.01
–
7.95(m,2h),7.93
–
7.88(m,2h),7.74(dd,j=3.6,1.2hz,1h),7.69
–
7.59(m,2h),7.47(dd,j=4.8,1.2hz,1h),7.16(dd,j=4.8,3.6hz,1h);
13
c nmr(100mhz,cdcl3):δ(ppm)152.0,148.0,145.1,131.5,131.3,131.1,129.6,128.7,128.3,128.2,128.1,127.1,127.0,125.5,124.0,122.5,117.4;hrms(esi):m/z[m+h]
+
calcd for c
17h12
ns:262.0685;found:262.0686。
[0099]
实施例34
[0100][0101]
在35ml密封管中加入1v(53.5mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=5/1)得到黄色固体产物4v(104mg,81%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)9.41
–
9.36(m,1h),8.98(d,j=8.8hz,1h),8.70(dd,j=4.8,1.6hz,1h),8.60(d,j=8.4hz,1h),8.53(dt,j=8.0,2.0hz,1h),8.06
–
7.97(m,3h),7.95
–
7.91(m,1h),7.72
–
7.63(m,2h),7.48
–
7.43(m,1h);
13
c nmr(100mhz,cdcl3):δ(ppm)154.0,150.1,148.7,148.3,134.8,134.7,131.7(3),131.7(0),131.3,129.4,128.7,128.3,127.4,127.2,124.5,123.7,122.7,118.4;hrms(esi):m/z[m+h]
+
calcd for c
18h13
n2:257.1073;found:257.1073。
[0102]
实施例35
[0103][0104]
在35ml密封管中加入1w(78.5mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,
过硅胶柱分离(石油醚/乙酸乙酯=5/1)得到黄色固体产物4w(121mg,79%)。该化合物的表征数据如下:1hnmr(400mhz,cdcl3):δ(ppm)9.00(d,j=8.4hz,1h),8.95(dd,j=4.0,1.6hz,1h),8.65
–
8.59(m,3h),8.27(t,j=9.0hz,2h),8.14
–
8.09(m,2h),8.02(d,j=9.2hz,1h),7.97
–
7.93(m,1h),7.72
–
7.63(m,2h),7.44(q,j=4.0hz,1h);
13
c nmr(100mhz,cdcl3):δ(ppm)155.7,151.0,148.7,148.3,137.3,136.8,131.7(1),131.6(6),131.2,130.0,129.5,128.7,128.6,128.5,128.3,127.3,127.2,126.6,124.3,122.7,121.5,118.8;hrms(esi):m/z[m+h]
+
calcd for c
22h15
n2:307.1230;found:307.1231。
[0105]
实施例36
[0106][0107]
在35ml密封管中加入1x(78.5mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=5/1)得到黄色固体产物4x(96.4mg,63%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)9.15(d,j=8.8hz,1h),9.08(d,j=4.4hz,1h),8.72(d,j=8.0hz,1h),8.26
–
8.22(m,2h),8.13
–
8.07(m,2h),8.01(dd,j=8.0,1.2hz,1h),7.90(d,j=8.4hz,1h),7.80
–
7.76(m,2h),7.75
–
7.70(m,1h),7.68(d,j=4.4hz,1h),7.60
–
7.55(m,1h);
13
c nmr(100mhz,cdcl3):δ(ppm)156.1,150.1,148.9,148.1,146.3,131.9,131.7,131.5,129.9,129.5,129.4,128.8,128.2,127.7,127.4,127.2,126.1,125.8,124.7,122.8,122.5,121.7;hrms(esi):m/z[m+h]
+
calcd for c
22h15
n2:307.1230;found:307.1232。
[0108]
实施例37
[0109][0110]
在35ml密封管中加入1y(73mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4y(99mg,67%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.96(d,j=8.8hz,1h),8.58(d,j=8.4hz,1h),8.13
–
8.10(m,1h),8.07(d,j=9.2hz,1h),7.99(d,j=9.2hz,1h),7.94
–
7.90(m,1h),7.70
–
7.60(m,5h),7.39
–
7.34(m,1h),7.30
–
7.26(m,1h);
13
c nmr(100mhz,cdcl3):δ(ppm)155.5,155.1,148.5,148.2,131.7,131.4(0),131.3(6),129.5,128.8,128.7,128.2,127.3,127.2,125.4,124.7,123.2,122.7,121.7,118.0,111.7,105.8;hrms(esi):m/z[m+h]
+
calcd for c
21h14
no:296.1070;found:296.1069。
[0111]
实施例38
[0112][0113]
在35ml密封管中加入1z(81mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4z(124.4mg,80%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.96(d,j=8.4hz,1h),8.84(d,j=7.6hz,1h),8.61(d,j=8.0hz,1h),8.11(d,j=8.8hz,1h),8.01(d,j=9.2hz,1h),7.96
–
7.91(m,4h),7.72
–
7.62(m,2h),7.51(ddd,j=8.4,7.2,1.2hz,1h),7.45
–
7.41(m,1h);
13
c nmr(100mhz,cdcl3):δ(ppm)154.1,148.0,140.9,137.4,136.4,131.6,131.3,131.0,129.6,128.7,128.4,127.3,127.1(2),127.1(0),124.8(0),124.7(8),124.7,123.8,122.6(4),122.5(6),120.7;hrms(esi):m/z[m+h]
+
calcd for c
21h14
ns:312.0841;found:312.0842。
[0114]
实施例39
[0115][0116]
在35ml密封管中加入1aa(79.5mg,0.5mmol)、2a(71.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=30/1)得到黄色固体产物4aa(88mg,57%)。该化合物的表征数据如下:1hnmr(400mhz,cdcl3):δ(ppm)8.90(d,j=8.8hz,1h),8.73
–
8.68(m,1h),8.59(d,j=8.4hz,1h),8.08(d,j=9.2hz,1h),7.99
–
7.92(m,3h),7.83(s,1h),7.70
–
7.65(m,1h),7.63
–
7.58(m,1h),7.42
–
7.38(m,1h),7.36
–
7.31(m,2h),3.90(s,3h);
13
cnmr(100mhz,cdcl3):δ(ppm)154.8,148.3,137.9,131.3,130.7,130.5,129.9,129.7,128.6(4),128.5(6),126.8,126.4,126.3,122.6,122.4,122.3,122.0,120.9,119.0,116.0,109.5,33.2;hrms(esi):m/z[m+h]
+
calcd for c
22h17
n2:309.1386;found:309.1388。
[0117]
实施例40
[0118][0119]
在35ml密封管中加入1a(53mg,0.5mmol)、2b(111mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,
过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4ab(142mg,85%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.87(d,j=8.8hz,1h),8.42(d,j=8.8hz,1h),8.21
–
8.18(m,2h),8.11
–
8.02(m,2h),7.99(d,j=8.4hz,1h),7.87(d,j=9.2hz,1h),7.74(dd,j=8.8,2.0hz,1h),7.58
–
7.50(m,2h),7.50
–
7.45(m,1h);
13
c nmr(100mhz,cdcl3):δ(ppm)157.2,148.0,139.2,133.0,131.3,130.8,130.2,129.9,129.7,129.4,128.9,128.2,127.4,124.3,123.8,121.1,119.1;hrms(esi):m/z[m+h]
+
calcd for c
19h13
brn:334.0226;found:334.0229。
[0120]
实施例41
[0121][0122]
在35ml密封管中加入1a(53mg,0.5mmol)、2c(86mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4ac(135mg,95%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.79(d,j=8.8hz,1h),8.43(d,j=8.8hz,1h),8.19
–
8.15(m,2h),8.04(d,j=9.2hz,1h),7.92
–
7.84(m,2h),7.55
–
7.49(m,2h),7.47
–
7.42(m,1h),7.30
–
7.25(m,2h),3.94(s,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)158.7,155.8,147.2,139.5,133.0,130.9,130.4,129.1,129.0,128.8,127.3,124.2,124.1,123.7,118.8,117.7,108.6,55.4;hrms(esi):m/z[m+h]
+
calcd for c
20h16
no:286.1226;found:286.1228。
[0123]
实施例42
[0124][0125]
在35ml密封管中加入1a(53mg,0.5mmol)、2d(100.5mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4ad(130mg,83%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.94(d,j=8.8hz,1h),8.62(d,j=1.6hz,1h),8.60(d,j=8.8hz,1h),8.26(dd,j=8.8,1.6hz,1h),8.22
–
8.19(m,2h),8.10(d,j=9.2hz,1h),8.03(d,j=9.2hz,1h),8.00(d,j=8.8hz,1h),7.57
–
7.52(m,2h),7.51
–
7.46(m,1h),4.00(s,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)166.9,157.7,148.9,139.1,132.6,132.0,131.2,130.9(5),130.8(7),129.5(2),129.4(8),128.9,128.4,127.5,126.8,123.5,122.8,119.0,52.3;hrms(esi):m/z[m+h]
+
calcd for c
21h16
no2:314.1176;found:314.1179。
[0126]
实施例43
[0127][0128]
在35ml密封管中加入1a(53mg,0.5mmol)、2e(79mg,0.5mmol)、3a(151.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4ae(84mg,62%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.83(d,j=8.8hz,1h),8.41(d,j=8.4hz,1h),8.21
–
8.15(m,2h),7.98(d,j=9.2hz,1h),7.95(d,j=8.4hz,1h),7.79(d,j=9.2hz,1h),7.53(t,j=7.6hz,2h),7.47
–
7.42(m,1h),7.13
–
7.07(m,2h),3.98(s,2h);13c nmr(100mhz,cdcl3):δ(ppm)155.3,146.9,145.6,139.7,133.3,130.5,130.1,129.0,128.9,128.8,127.2,124.6,124.0,122.2,118.8,117.3,111.1;hrms(esi):m/z[m+h]+calcd for c19h15n2:271.1230;found:271.1233。
[0129]
实施例44
[0130][0131]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3b(214.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4af(117mg,87%)。该化合物的表征数据如下:1hnmr(400mhz,cdcl3):δ(ppm)8.70(s,1h),8.55(d,j=8.0hz,1h),8.00(d,j=9.2hz,1h),7.89
–
7.85(m,2h),7.65
–
7.60(m,3h),7.60
–
7.56(m,1h),7.51
–
7.46(m,2h),7.44
–
7.40(m,1h),2.52(s,3h);13c nmr(100mhz,cdcl3):δ(ppm)159.2,146.1,140.6,132.1,131.6,129.9,129.1,129.0,128.9,128.5,128.2,128.1,128.0,126.9,126.7,124.2,122.5,20.8;hrms(esi):m/z[m+h]+calcd for c20h16n:270.1277;found:270.1279。
[0132]
实施例45
[0133][0134]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3c(341mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,
过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4ag(117.3mg,79%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.83(s,1h),8.67(d,j=8.0hz,1h),8.03(d,j=8.8hz,1h),7.96
–
7.91(m,2h),7.72
–
7.61(m,2h),7.61
–
7.57(m,2h),7.53
–
7.47(m,2h),7.47
–
7.42(m,1h),2.86(t,j=7.6hz,2h),1.70
–
1.60(m,2h),0.90(t,j=7.2hz,3h);
13
cnmr(100mhz,cdcl3):δ(ppm)159.7,146.0,140.8,133.8,131.7,131.2,130.1,129.3,128.9,128.7,128.3,128.2,128.0,127.0,126.8,124.4,122.6,35.1,24.2,13.9;hrms(esi):m/z[m+h]
+
calcd for c
22h20
n:298.1590;found:298.1591。
[0135]
实施例46
[0136][0137]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3d(341mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4ah(96.5mg,65%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.94(s,1h),8.71(d,j=8.0hz,1h),8.02(d,j=8.8hz,1h),7.96
–
7.91(m,2h),7.74
–
7.69(m,1h),7.67
–
7.62(m,1h),7.59
–
7.55(m,2h),7.53
–
7.48(m,2h),7.48
–
7.43(m,1h),3.40
–
3.30(m,1h),1.34(d,j=6.8hz,6h);
13
cnmr(100mhz,cdcl3):δ(ppm)159.2,145.8,140.8,140.2,131.7,130.2,129.4,128.9,128.7,128.3,128.2,128.0,127.9,127.0,126.8,124.6,122.6,29.4,24.4;hrms(esi):m/z[m+h]
+
calcd for c
22h20
n:298.1590;found:298.1587。
[0138]
实施例47
[0139][0140]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3e(404mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4ai(132mg,85%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.82(s,1h),8.67(d,j=8.0hz,1h),8.02(d,j=9.2hz,1h),7.95
–
7.90(m,2h),7.72
–
7.67(m,1h),7.66
–
7.61(m,1h),7.61
–
7.57(m,2h),7.53
–
7.47(m,2h),7.47
–
7.42(m,1h),2.88(t,j=7.6hz 2h),1.64
–
1.56(m,2h),1.35
–
1.26(m,2h),0.85(t,j=7.6hz,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)159.6,146.0,140.8,134.1,131.7,131.1,130.1,129.3,128.9,128.7,128.3,128.2,128.0,127.0,126.8,
124.4,122.6,33.3,32.8,22.4,13.8;hrms(esi):m/z[m+h]
+
calcd for c
23h22
n:312.1747;found:312.1746。
[0141]
实施例48
[0142][0143]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3f(467mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4aj(120mg,74%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.83(s,1h),8.67(d,j=8.4hz,1h),8.03(d,j=9.2hz,1h),7.94(d,j=9.2hz,2h),7.72
–
7.62(m,2h),7.61
–
7.57(m,2h),7.53
–
7.47(m,2h),7.47
–
7.42(m,1h),2.87(t,j=8.0hz,2h),1.66
–
1.57(m,2h),1.29
–
1.22(m,4h),0.83(t,j=7.2hz,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)159.7,146.0,140.8,134.1,131.7,131.2,130.1,129.3,128.9,128.7,128.3,128.2,128.0,127.0,126.8,124.4,122.6,33.1,31.5,30.8,22.3,13.9;hrms(esi):m/z[m+h]
+
calcd for c
24h24
n:326.1903;found:326.1904。
[0144]
实施例49
[0145][0146]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3g(530.5mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4ak(152.5mg,90%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.80(s,1h),8.64(d,j=8.0hz,1h),8.02(d,j=9.2hz,1h),7.93
–
7.88(m,2h),7.70
–
7.65(m,1h),7.63
–
7.57(m,3h),7.52
–
7.46(m,2h),7.46
–
7.41(m,1h),2.85(t,j=8.0hz,2h),1.63
–
1.55(m,2h),1.30
–
1.18(m,6h),0.83(t,j=6.8hz,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)159.6,145.9,140.8,134.1,131.7,131.1,130.0,129.3,128.9,128.6,128.2,128.1,128.0,127.0,126.8,124.3,122.6,33.0,31.4,31.0,29.0,22.4,14.0;hrms(esi):m/z[m+h]
+
calcd for c
25h26
n:340.2060;found:340.2062。
[0147]
实施例50
[0148][0149]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3h(593.6mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4al(123.5mg,70%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.83(s,1h),8.68(d,j=8.0hz,1h),8.03(d,j=8.8hz,1h),7.94(d,j=8.8hz,2h),7.73
–
7.68(m,1h),7.67
–
7.62(m,1h),7.61
–
7.57(m,2h),7.53
–
7.47(m,2h),7.47
–
7.42(m,1h),2.88(t,j=7.6hz,2h),1.65
–
1.57(m,2h),1.30
–
1.17(m,8h),0.85(t,j=7.2hz,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)159.6,146.0,140.8,134.2,131.7,131.2,130.1,129.3,128.9,128.7,128.3,128.2,128.0,127.0,126.8,124.4,122.6,33.1,31.6,31.1,29.3,28.9,22.6,14.1;hrms(esi):m/z[m+h]
+
calcd for c
26h28
n:354.2216;found:354.2212。
[0150]
实施例51
[0151][0152]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3i(656.7mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4am(156mg,85%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.82(s,1h),8.67(d,j=8.0hz,1h),8.02(d,j=9.2hz,1h),7.93(d,j=9.2hz,2h),7.72
–
7.67(m,1h),7.66
–
7.61(m,1h),7.61
–
7.57(m,2h),7.52
–
7.47(m,2h),7.47
–
7.42(m,1h),2.87(t,j=8.0hz,2h),1.65
–
1.56(m,2h),1.28
–
1.17(m,10h),0.86(t,j=7.2hz,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)159.7,146.0,140.8,134.1,131.8,131.1,130.1,129.3,128.9,128.7,128.3,128.2,128.0,127.0,126.8,124.4,122.6,33.1,31.8,31.1,29.3,29.2,29.1,22.6,14.1;hrms(esi):m/z[m+h]
+
calcd for c
27h30
n:368.2373;found:368.2373。
[0153]
实施例52
[0154][0155]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3j(720mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4an(137mg,72%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.84(s,1h),8.68(d,j=8.0hz,1h),8.03(d,j=9.2hz,1h),7.94(d,j=8.8hz,2h),7.73
–
7.69(m,1h),7.67
–
7.63(m,1h),7.62
–
7.57(m,2h),7.53
–
7.48(m,2h),7.47
–
7.42(m,1h),2.88(t,j=8.0hz,2h),1.63
–
1.56(m,2h),1.30
–
1.19(m,12h),0.87(t,j=7.2hz,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)159.7,146.0,140.8,134.2,131.8,131.2,130.1,129.3,128.9,128.7,128.3,128.2,128.0,127.0,126.8,124.4,122.6,33.1,31.8,31.1,29.4,29.33,29.26,29.2,22.6,14.1;hrms(esi):m/z[m+h]
+
calcd for c
28h32
n:382.2529;found:382.2527。
[0156]
实施例53
[0157][0158]
在35ml密封管中加入1a(53mg,0.5mmol)、2a(71.5mg,0.5mmol)、3k(783mg,1.5mmol)、碘化铵(108.7mg,0.75mmol)、二叔丁基过氧化物(146mg,1mmol)和氯苯(2ml),然后置于120℃金属浴中搅拌8h。加入50ml水淬灭反应,用乙酸乙酯萃取(50ml
×
3),之后有机相用质量浓度为10%的na2s2o3溶液和饱和食盐水依次洗涤,无水硫酸钠干燥。过滤,旋干,过硅胶柱分离(石油醚/乙酸乙酯=100/1)得到黄色固体产物4ao(134mg,68%)。该化合物的表征数据如下:1h nmr(400mhz,cdcl3):δ(ppm)8.82(s,1h),8.67(d,j=8.0hz,1h),8.02(d,j=9.2hz,1h),7.93(d,j=9.2hz,2h),7.72
–
7.67(m,1h),7.66
–
7.62(m,1h),7.61
–
7.57(m,2h),7.52
–
7.47(m,2h),7.46
–
7.41(m,1h),2.87(t,j=8.0hz,2h),1.65
–
1.56(m,2h),1.29
–
1.20(m,14h),0.87(t,j=6.8hz,3h);
13
c nmr(100mhz,cdcl3):δ(ppm)159.7,146.0,140.8,134.1,131.8,131.2,130.1,129.3,128.9,128.7,128.3,128.2,128.0,127.0,126.8,124.4,122.6,33.1,31.9,31.1,29.5,29.4,29.32,29.27,29.2,22.6,14.1;hrms(esi):m/z[m+h]
+
calcd for c
29h34
n:396.2686;found:396.2686。
[0159]
以上实施例描述了本发明的基本原理、主要特征及优点,本行业的技术人员应该了解,本发明不受上述实施例的限制,上述实施例和说明书中描述的只是说明本发明的原
理,在不脱离本发明原理的范围下,本发明还会有各种变化和改进,这些变化和改进均落入本发明保护的范围内。
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1