一种氮杂环卡宾催化的选择性酰化核苷类衍生物的制备方法及其用途
本发明涉及一种氮杂环卡宾催化的选择性酰化核苷类衍生物的制备方法及用途
背景技术:
1、核苷和核苷酸是生命分子的重要组成部分,对生物体的遗传和能量供应至关重要。核苷类似物作为有效药物在各种疾病的治疗中发挥了关键作用。尽管在核苷药物开发方面取得了巨大成功,但其临床应用经常受到生物利用度低、药代动力学特性不理想以及高剂量导致的危险副作用的限制。优化核苷药物效率的有效策略之一是对这些生物活性核苷结构进行化学修饰,以实现具有改善的生物利用度和药代动力学特性的前药。核苷药物分子中oh基团的选择性酯化是药物优化的一类最有效的策略(chem.rev.100,4319-4348(2000);bioorg.med.chem.8,2681-2687(2000);j.am.chem.soc.110,7200-7205(2002))。
2、传统上,核苷分子上羟基的区域选择性单官能化需要用预保护/去保护程序。例如,利巴韦林中糖基部分的5’-oh基团的单官能化需要通过与当量的羰基化合物形成缩醛结构来预保护2’-oh基团和3’-oh基团,在5’-oh官能化和2’-oh和3’-oh基团的去保护之后,可以得到5’-oh单官能化产物(j.med.chem.60,1648-1661(2017))。类似地,利巴韦林2’-oh官能化通常涉及通过二氯硅烷试剂对3’-oh和5’-oh基团的预保护以及目标转化后的脱保护步骤(j.med.chem.43,1019-1028(2000))。酶催化方法可以直接区域选择性地获得5’-o-酰化利巴韦林衍生物,但利巴韦林及其类似物的其他oh基团上的位点选择性功能化尚未实现。据我们所知,利巴韦林3’-oh单官能化的简便和区域选择性方法尚未报道,尽管酶催化和跨金属催化可以产生除利巴韦林以外的几种3’-oh单官能化的核苷(j.org.chem.39,24-30(1974);biotechnol.tech.13,563-566(1999))。因此,开发可编程、经济、简便和无毒的核苷分子区域选择性功能化方法是非常有意义的。
技术实现思路
1、本发明的目的是为了设计合成结构新颖、底物普适性好和高区域选择性的单酰化核苷类衍生物,并进一步发掘其在生物活性方面的用途。
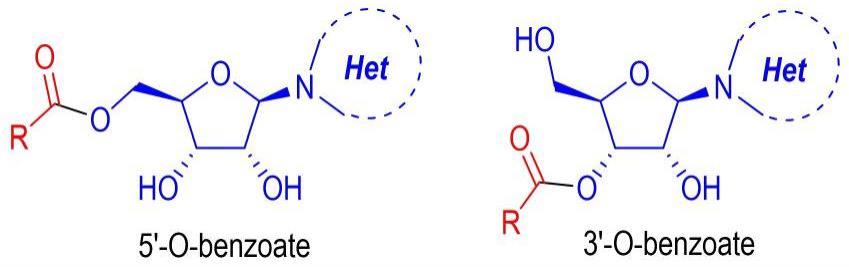
2、本发明的选择性酰化核苷类衍生物,如下述通式(1)表示:
3、
4、其中r为苯基、取代苯基、呋喃、噻吩、萘基、烯基苯、柠檬醛基、香草醛基、丙磺舒基、托灭酸基、2-甲基丙烯基或异丙基;
5、n-杂环为嘌呤、嘧啶碱基、5-甲基尿苷、腺苷或8-溴腺苷。
6、所述取代苯基原子为卤原子,三氟甲基,甲基,甲氧基。
7、所述卤原子为氟、氯或溴。
8、反应通式及过程如下:
9、
10、合成的衍生物如下:
11、substrate scope for the 5'-oh acylation reaction of nucleosidesa
12、
13、a反应条件:核苷(0.10mmol),rcho(0.20mmol),nhc c(0.01mmol),dbu(0.02mmol),dq(0.10mmol),硼酸(0.10mmol),dmf(1.0ml),在30℃条件下反应12h.b反应18小时.收率为2+3+4的总收率.r.r.为区域选择性.
14、substrate scope for the 3'-oh acylation reaction of nucleosidesa
15、
16、a反应条件:核苷(0.10mmol),rcho(0.20mmol),nhc e(0.01mmol),na2co3(0.02mmol),dq(0.10mmol),dmf/mecn(1.0ml),在50℃条件下反应2h.b反应3h.收率为2+3+4的总收率.r.r.为区域选择性.
17、合成的口服抗新冠药物方法如下:
18、
19、本发明的有益效果是:具有不同生物活性的核苷类化合物能有效地在氮杂环卡宾的催化作用下,高效的制备5’-o-酰化核苷衍生物和3’-o-酰化核苷衍生物等前药分子,并具有底物普适性好(62个化合物)、优异的产率和高区域映选择性(收率最高为82%,区域选择性>20:1)等优点。目前开发的核苷区域选择性单酯化的有机催化方法可作为高效合成对新型冠状病毒感染治疗至关重要药物的关键步骤。所得到的核苷单酯产品还显示出了对植物病毒有良好的抗病毒活性,并为未来开发抗病毒农药提供了关键信息。
20、具体制备实施方式
21、以下介绍本发明的实施例,介绍62个制备实施例
22、制备实施例1
23、制备((2r,3s,4r,5r)-5-(3-氨基甲酰基-1h-1,2,4-三唑-1-基)-3,4-二羟基四氢呋喃-2-基)苯甲酸甲酯(2):
24、
25、制备实施方法和条件如下:
26、在手套箱中,分别称取nhc催化剂c(0.01mmol,10mol%,3.6mg)、dbu(0.02mmol,20mol%,3.0μl)、dq(0.10mmol,100mol%,40.8mg)、ba-3(0.10mmol、100mol%、12.2mg)、利巴韦林(0.10mmol)和芳香醛(0.20mmol)加入配有磁力搅拌子的4.0ml反应瓶中,加入1.0mldmf溶液,轻轻晃动反应壁,使其充分混匀,30摄氏度搅拌反应12h。tlc监测反应完毕后旋干,干法上样,通过柱层析分离(洗脱剂极性二氯甲烷:甲醇=100:5),得到目标化合物2,称量后计算相应的产率,化合物通过核磁共振仪nmr和高分辨质谱仪hrms予以表征。
27、2h),7.85(s,1h),7.67(s,1h),7.66–7.62(m,1h),7.53(t,j=7.61hz,2h),5.96(d,j=2.67hz,1h),5.71(d,j=5.03hz,1h),5.44(d,j=5.98hz,1h),4.53–4.47(m,2h),4.44–4.41(m,1h),4.41–4.36(m,1h),4.29–4.24(m,1h).
28、13c nmr(101mhz,dmso)δ166.1,160.8,158.2,146.2,133.9,129.8,129.8,129.3,91.7,81.8,74.7,70.7,64.6.
29、hrms(esi,m/z)calcd.for c15h16n4o6na+:371.0962,found:371.0960.
30、制备实施例2
31、(2r,3s,4r,5r)-5-(3-氨基甲酰基-1h-1,2,4-三唑-1-基)-4-羟基-2-(羟甲基)四氢呋喃-3-基苯甲酸酯(3)
32、
33、制备实施方法和条件如下:
34、在手套箱中,分别称取nhc催化剂e(0.01mmol,10mol%,2.9mg)、na2co3(0.02mmol,20mol%,2.1mg)、dq(0.10mmol,100mol%,40.8mg)、利巴韦林(0.10mmol)和芳香醛(0.20mmol)加入配有磁力搅拌子的4.0ml反应瓶中,加入1.0mldmf:mecn=1:1溶液,轻轻晃动反应壁,使其充分混匀,50摄氏度搅拌反应2h。tlc监测反应完毕后旋干,干法上样,通过柱层析分离(洗脱剂极性二氯甲烷:甲醇=100:5),得到目标化合物3,称量后计算相应的产率,化合物通过核磁共振仪nmr和高分辨质谱仪hrms予以表征。
35、
36、white solid,70%yield,24.3mg;5:1(regioselective ratio);
37、1h nmr(400mhz,dmso)δ8.94(s,1h),8.09–8.05(m,2h),7.88(s,1h),7.73–7.69(m,1h),7.68(s,1h),7.60–7.56(m,2h),6.05(d,j=6.02hz,1h),6.01(d,j=5.09hz,1h),5.49(dd,j=5.14,3.87hz,1h),5.13(t,j=5.66hz,1h),4.83(q,j=5.41hz,1h),4.33(q,j=4.44hz,1h),3.72–3.62(m,2h).
38、13c nmr(101mhz,dmso)δ165.5,160.8,158.0,146.0,134.0,130.0,129.9,129.2,91.8,83.8,74.1,73.3,61.7.
39、hrms(esi,m/z)calcd.for c15h16n4o6na+:371.0962,found:371.0961.
40、制备实施例3
41、取代基r为4-ch3-ph,制备实施方法和条件同制备实施例1;
42、
43、ratio);
44、1h nmr(400mhz,dmso)δ8.84(s,1h),7.86(d,j=8.02hz,2h),7.84–7.82(m,1h),7.69(s,1h),7.33(d,j=7.88hz,2h),5.95(d,j=2.65hz,1h),5.70(d,j=5.02hz,1h),5.43(d,j=5.95hz,1h),4.52–4.44(m,2h),4.41(td,j=4.83,2.67hz,1h),4.37(dd,j=12.16,5.13hz,1h),4.28–4.23(m,1h),2.37(s,3h).
45、13c nmr(101mhz,dmso)δ166.1,160.8,158.2,146.2,144.1,129.9,129.8,127.1,91.7,81.8,74.7,70.7,64.4,21.7.
46、hrms(esi,m/z)calcd.for c16h18n4o6na+:385.1119,found:385.1114.
47、制备实施例4
48、取代基r为4-och3-ph,制备实施方法和条件同制备实施例1;
49、
50、3,4-dihydroxytetrahydrofuran-2-yl)methyl 4-methoxybenzoate(7):
51、white solid,72%yield,27.4mg;19:1(regioselective ratio);
52、1h nmr(400mhz,dmso)δ8.85(d,j=1.38hz,1h),7.92(d,j=8.53hz,2h),7.86(s,1h),7.70(s,1h),7.05(d,j=8.49hz,2h),5.95(d,j=2.60hz,1h),5.70(d,j=4.95hz,1h),5.42(d,j=5.97hz,1h),4.49(d,j=5.91hz,1h),4.47–4.40(m,2h),4.35(dd,j=12.26,4.89hz,1h),4.25(dt,j=9.37,3.68hz,1h),3.83(s,3h).
53、13c nmr(101mhz,dmso)δ165.8,163.7,160.9,158.2,146.2,131.9,122.0,114.6,91.6,81.9,74.6,70.6,64.1,55.9.
54、hrms(esi,m/z)calcd.for c16h18n4o7na+:401.1068,found:401.1059.
55、制备实施例5
56、取代基r为4-cf3-ph,制备实施方法和条件同制备实施例1;
57、ratio);
58、1h nmr(400mhz,dmso)δ8.88(s,1h),8.23(d,j=8.10hz,2h),7.93(d,j=8.19hz,2h),7.90(s,1h),7.75(s,1h),5.97(d,j=2.82hz,1h),5.70(d,j=5.03hz,1h),5.44(d,j=5.84hz,1h),4.59–4.47(m,3h),4.47–4.40(m,1h),4.32–4.25(m,1h).
59、13c nmr(101mhz,dmso)δ165.1,160.9,158.3,146.4,133.6,133.4(d,j=31.8hz),130.8,126.4(d,j=4.0hz),124.2(d,j=273.2hz),91.5,81.7,74.7,70.6,64.8.
60、19f nmr(377mhz,dmso)δ-61.62.
61、hrms(esi,m/z)calcd.for c16h15f3n4o6na+:439.0836,found:439.0825.
62、制备实施例6
63、取代基r为4-f-ph,制备实施方法和条件同制备实施例1;
64、
65、1h nmr(400mhz,dmso)δ8.86(s,1h),8.11–8.04(m,2h),7.89(s,1h),7.72(s,1h),7.40–7.32(m,2h),5.96(d,j=2.61hz,1h),5.70(d,j=5.00hz,1h),5.43(d,j=5.97hz,1h),4.55–4.46(m,2h),4.44–4.37(m,2h),4.29–4.23(m,1h).
66、13c nmr(101mhz,dmso)δ165.6(d,j=251.2hz),165.2,160.9,158.2,146.3,132.8(d,j=9.8hz),126.5(d,j=2.9hz),116.4(d,j=22.3hz),91.5,81.7,74.7,70.5,64.4.
67、19f nmr(377mhz,dmso)δ-105.87.
68、hrms(esi,m/z)calcd.for c15h15fn4o6na+:389.0868,found:389.0861.
69、制备实施例7
70、取代基r为4-cl-ph,制备实施方法和条件同制备实施例1;
71、ratio);
72、1h nmr(400mhz,dmso)δ8.86(s,1h),8.05–8.00(m,2h),7.90(s,1h),7.74(s,1h),7.64–7.59(m,2h),5.96(d,j=2.69hz,1h),5.70(d,j=5.00hz,1h),5.43(d,j=6.00hz,1h),4.55–4.50(m,1h),4.48(dd,j=12.10,2.85hz,1h),4.44–4.38(m,2h),4.29–4.23(m,1h).
73、13c nmr(101mhz,dmso)δ165.4,160.9,158.2,146.3,138.8,131.8,129.5,128.7,91.5,81.7,74.7,70.5,64.5.
74、hrms(esi,m/z)calcd.for c15h15cln4o6na+:405.0572found:405.0563.
75、制备实施例8
76、取代基r为4-br-ph,制备实施方法和条件同制备实施例1;
77、ratio);
78、1h nmr(400mhz,dmso)δ8.87(s,1h),7.97–7.92(m,2h),7.90(s,1h),7.78–7.73(m,3h),5.96(d,j=2.69hz,1h),5.70(d,j=5.02hz,1h),5.43(d,j=5.95hz,1h),4.54–4.50(m,1h),4.49–4.45(m,1h),4.44–4.39(m,2h),4.28–4.23(m,1h).
79、13c nmr(101mhz,dmso)δ165.5,160.9,158.2,146.4,132.5,131.9,129.0,128.0,91.5,81.7,74.7,70.5,64.5.
80、hrms(esi,m/z)calcd.for c15h15brn4o6na+:449.0067,found:449.0060.
81、制备实施例9
82、取代基r为3-och3-ph,制备实施方法和条件同制备实施例1;
83、ratio);
84、1h nmr(400mhz,dmso)δ8.84(s,1h),7.82(s,1h),7.66(s,1h),7.57(dt,j=7.71,1.24hz,1h),7.45(t,j=7.94hz,1h),7.42(dd,j=2.69,1.51hz,1h),7.25–7.20(m,1h),5.95(d,j=2.70hz,1h),5.72(d,j=4.92hz,1h),5.44(d,j=5.72hz,1h),4.51(dd,j=12.11,3.28hz,1h),4.47–4.41(m,2h),4.41–4.36(m,1h),4.27(td,j=5.78,3.24hz,1h),3.80(s,3h).
85、13c nmr(101mhz,dmso)δ165.9,160.8,159.7,158.1,146.1,131.2,130.6,122.1,119.9,114.3,91.8,81.9,74.7,70.9,65.1,55.8.
86、hrms(esi,m/z)calcd.for c16h18n4o7na+:401.1068,found:401.1061.
87、制备实施例10
88、取代基r为3-f-ph,制备实施方法和条件同制备实施例1;
89、
90、1h nmr(400mhz,dmso)δ8.85(s,1h),7.87–7.81(m,2h),7.65(pd,j=3.74,3.07,1.80hz,2h),7.62–7.56(m,1h),7.56–7.49(m,1h),5.95(d,j=2.74hz,1h),5.72(d,j=5.06hz,1h),5.44(d,j=5.97hz,1h),4.53(dd,j=12.06,3.27hz,1h),4.50–4.45(m,1h),4.41(dt,j=7.78,5.18hz,2h),4.27(td,j=5.75,3.16hz,1h).
91、13c nmr(101mhz,dmso)δ165.0,162.4(d,j=245.2hz),160.8,158.2,146.2,132.1(d,j=7.4hz),131.7(d,j=8.0hz),126.1(d,j=2.9hz),120.9(d,j=20.9hz),116.2(d,j=22.7hz),91.7,81.8,74.7,70.8,65.2.
92、19f nmr(377mhz,dmso)δ-112.11.
93、hrms(esi,m/z)calcd.for c15h15fn4o6na+:389.0868,found:389.0857.
94、制备实施例11
95、取代基r为3-cl-ph,制备实施方法和条件同制备实施例1;
96、
97、1h nmr(400mhz,dmso)δ8.85(s,1h),7.96(dt,j=7.79,1.33hz,1h),7.88(t,j=1.88hz,1h),7.84(s,1h),7.76–7.72(m,1h),7.65(s,1h),7.58(t,j=7.93hz,1h),5.95(d,j=2.71hz,1h),5.71(d,j=5.04hz,1h),5.45(d,j=6.00hz,1h),4.53(dd,j=12.09,3.30hz,1h),4.47(td,j=6.21,4.80hz,1h),4.44–4.37(m,2h),4.27(td,j=5.88,3.22hz,1h).
98、13c nmr(101mhz,dmso)δ164.9,160.8,158.2,146.2,133.9,133.7,131.9,131.5,129.2,128.5,91.8,81.7,74.7,70.8,65.3.
99、hrms(esi,m/z)calcd.for c15h15cln4o6na+:405.0572,found:405.0560.
100、制备实施例12
101、取代基r为3-br-ph,制备实施方法和条件同制备实施例1;
102、
103、white solid,62%yield,26.5mg;20:1(regioselective ratio);
104、1h nmr(400mhz,dmso)δ8.85(s,1h),8.05–7.98(m,2h),7.90–7.86(m,1h),7.84(s,1h),7.66(s,1h),7.52(t,j=7.89hz,1h),5.95(d,j=2.73hz,1h),5.71(d,j=5.05hz,1h),5.45(d,j=5.94hz,1h),4.53(dd,j=12.08,3.30hz,1h),4.50–4.44(m,1h),4.44–4.37(m,2h),4.30–4.24(m,1h).
105、13c nmr(101mhz,dmso)δ164.8,160.8,158.2,146.2,136.6,132.1,131.7,128.9,122.3,91.8,81.7,74.7,70.8,65.4.
106、hrms(esi,m/z)calcd.for c15h15brn4o6na+:449.0067,found:449.0061.
107、制备实施例13
108、取代基r为2-ch3-ph,制备实施方法和条件同制备实施例1;
109、
110、1h nmr(400mhz,dmso)δ8.84(s,1h),7.84(d,j=1.59hz,1h),7.83–7.81(m,1h),7.65(s,1h),7.47(td,j=7.51,1.47hz,1h),7.32(t,j=8.12hz,2h),5.94(d,j=2.48hz,1h),5.71(d,j=4.72hz,1h),5.44(d,j=5.50hz,1h),4.51(dd,j=11.98,3.32hz,1h),4.45–4.38(m,2h),4.34(dd,j=12.00,5.98hz,1h),4.26(td,j=5.85,3.25hz,1h),2.49(s,3h).
111、13c nmr(101mhz,dmso)δ167.0,160.8,158.1,146.1,139.8,132.7,132.1,130.8,129.4,126.6,91.8,81.9,74.6,70.9,64.8,21.5.
112、hrms(esi,m/z)calcd.for c16h18n4o6na+:385.1119,found:385.1110.
113、制备实施例14
114、取代基r为2-f-ph,制备实施方法和条件同制备实施例1;
115、
116、1h nmr(400mhz,dmso)δ8.84(s,1h),7.92(td,j=7.79,1.92hz,1h),7.84(s,1h),7.72–7.67(m,1h),7.66(d,j=1.87hz,1h),7.39–7.30(m,2h),5.95(d,j=2.66hz,1h),5.73(d,j=4.87hz,1h),5.45(d,j=5.64hz,1h),4.55(dd,j=12.10,3.18hz,1h),4.47–4.40(m,2h),4.40–4.36(m,1h),4.26(td,j=5.81,3.11hz,1h).
117、13c nmr(101mhz,dmso)δ163.5(d,j=3.6hz),161.5(d,j=257.9hz),160.8,158.1,146.0,135.8(d,j=9.3hz),132.4,125.3(d,j=3.9hz),118.3(d,j=9.4hz),117.5(d,j=21.8hz),91.8,81.8,74.6,70.8,65.1.
118、19f nmr(377mhz,dmso)δ-110.72.
119、hrms(esi,m/z)calcd.for c15h15fn4o6na+:389.0869,found:389.0862.
120、制备实施例15
121、取代基r为2-cl-ph,制备实施方法和条件同制备实施例1;
122、
123、1h nmr(400mhz,dmso)δ8.84(s,1h),7.87(d,j=1.42hz,1h),7.86–7.84(m,1h),7.66(s,1h),7.60–7.55(m,2h),7.51–7.46(m,1h),5.94(d,j=2.58hz,1h),5.71(d,j=4.76hz,1h),5.45(d,j=5.43hz,1h),4.57(dd,j=12.05,3.28hz,1h),4.45–4.39(m,2h),4.39–4.34(m,1h),4.26(td,j=5.76,3.17hz,1h).
124、13c nmr(101mhz,dmso)δ165.1,160.8,158.2,146.1,133.8,132.5,131.8,131.3,129.9,128.0,91.8,81.8,74.6,70.9,65.4.
125、hrms(esi,m/z)calcd.for c15h15cln4o6na+:405.0572,found:405.0564.
126、制备实施例16
127、取代基r为2-br-ph,制备实施方法和条件同制备实施例1;
128、
129、1h nmr(400mhz,dmso)δ8.84(s,1h),7.88–7.84(m,1h),7.82(dd,j=7.50,1.97hz,1h),7.75(dd,j=7.74,1.49hz,1h),7.66(s,1h),7.55–7.46(m,2h),5.94(d,j=2.58hz,1h),5.71(d,j=4.83hz,1h),5.46(d,j=5.42hz,1h),4.57(dd,j=12.00,3.27hz,1h),4.45–4.39(m,2h),4.36(dd,j=11.96,5.90hz,1h),4.26(td,j=5.79,3.20hz,1h).
130、13c nmr(101mhz,dmso)δ165.7,160.8,158.2,146.1,134.5,133.8,132.1,131.7,128.5,120.8,91.8,81.9,74.6,70.9,65.6.
131、hrms(esi,m/z)calcd.for c15h15brn4o6na+:449.0067,found:449.0059.
132、制备实施例17
133、取代基r为萘基,制备实施方法和条件同制备实施例1;
134、ratio);
135、1h nmr(400mhz,dmso)δ8.88(s,1h),8.60(s,1h),8.16(d,j=8.10hz,1h),8.07–7.98(m,3h),7.87(s,1h),7.71–7.65(m,2h),7.65–7.59(m,1h),5.98(d,j=2.68hz,1h),5.74(d,j=5.01hz,1h),5.48(d,j=5.90hz,1h),4.60(dd,j=12.12,3.19hz,1h),4.53–4.46(m,2h),4.46–4.43(m,1h),4.36–4.30(m,1h).
136、13c nmr(101mhz,dmso)δ166.2,160.9,158.2,146.1,135.6,132.5,131.1,129.9,129.1,129.0,128.1,127.4,127.2,125.4,91.9,82.0,74.7,70.9,65.3.
137、hrms(esi,m/z)calcd.for c19h18n4o6na+:421.1119,found:421.1115.
138、制备实施例18
139、取代基r为呋喃,制备实施方法和条件同制备实施例1;
140、
141、1h nmr(400mhz,dmso)δ8.85(s,1h),7.99–7.95(m,1h),7.87–7.82(m,1h),7.69–7.63(m,1h),7.40(dd,j=3.54,0.87hz,1h),6.67(dd,j=3.54,1.72hz,1h),5.95(d,j=2.21hz,1h),5.76(s,1h),5.49(d,j=5.08hz,1h),4.50(dd,j=12.03,3.08hz,1h),4.43–4.39(m,2h),4.36(dd,j=12.09,5.79hz,1h),4.27–4.22(m,1h).
142、13c nmr(101mhz,dmso)δ160.9,158.1,158.1,148.3,146.0,143.9,119.5,112.9,91.9,82.0,74.7,70.8,64.7.
143、hrms(esi,m/z)calcd.for c13h14n4o7na+:361.0755,found:361.0749.
144、制备实施例19
145、取代基r为噻吩,制备实施方法和条件同制备实施例1;
146、
147、1h nmr(400mhz,dmso)δ8.83(s,1h),7.95(dd,j=4.99,1.30hz,1h),7.87(dd,j=3.75,1.34hz,1h),7.83(s,1h),7.65(s,1h),7.20(dd,j=4.96,3.73hz,1h),5.94(d,j=2.60hz,1h),5.71(d,j=4.92hz,1h),5.44(d,j=5.71hz,1h),4.50(dd,j=12.08,3.09hz,1h),4.46–4.38(m,2h),4.38–4.33(m,1h),4.27–4.21(m,1h).
148、13c nmr(101mhz,dmso)δ161.8,160.8,158.1,146.0,134.8,134.6,133.0,129.0,91.8,81.9,74.7,70.8,65.0.
149、hrms(esi,m/z)calcd.for c13h14n4o6sna+:377.0526,found:377.0516.
150、制备实施例20
151、取代基r为烯基苯,制备实施方法和条件同制备实施例1;
152、
153、ratio);
154、1h nmr(400mhz,dmso)δ8.85(s,1h),7.87(s,1h),7.76–7.70(m,2h),7.64(d,j=15.24hz,2h),7.44(q,j=3.73hz,3h),6.62(d,j=16.08hz,1h),5.93(d,j=2.37hz,1h),5.70(d,j=4.54hz,1h),5.43(d,j=5.15hz,1h),4.48–4.37(m,3h),4.28–4.17(m,2h).
155、13c nmr(101mhz,dmso)δ166.5,160.8,158.0,146.0,145.4,134.4,131.0,129.4,128.9,118.1,92.0,82.2,74.7,71.0,64.7.
156、hrms(esi,m/z)calcd.for c17h18n4o6na+:397.1119,found:397.1113.
157、制备实施例21
158、取代基r为吡啶,制备实施方法和条件同制备实施例1;
159、
160、white solid,68%yield,23.7mg;16:1(regioselective ratio);
161、1h nmr(400mhz,dmso)δ8.86(s,1h),8.83–8.79(m,2h),7.91–7.85(m,3h),7.71(s,1h),5.97(d,j=2.60hz,1h),5.73(s,1h),5.45(s,1h),4.57–4.51(m,2h),4.47–4.39(m,2h),4.30–4.25(m,1h).
162、13c nmr(101mhz,dmso)δ165.1,160.9,158.3,151.4,146.3,137.0,123.1,91.5,81.5,74.7,70.5,64.9.
163、hrms(esi,m/z)calcd.for c14h15n5o6na+:372.0915,found:372.0910.
164、制备实施例22
165、取代基r为香草醛骨架,制备实施方法和条件同制备实施例1;
166、
167、white solid,42%yield,16.4mg;10:1(regioselective ratio);
168、1h nmr(400mhz,dmso)δ9.98(s,1h),8.84(s,1h),7.87(s,1h),7.66(s,1h),7.49(dd,j=8.26,2.02hz,1h),7.41(d,j=2.01hz,1h),6.88(d,j=8.29hz,1h),5.93(d,j=2.24hz,1h),5.72(d,j=4.53hz,1h),5.45(d,j=5.22hz,1h),4.47–4.43(m,1h),4.42–4.38(m,2h),4.34(dd,j=12.05,5.79hz,1h),4.27–4.22(m,1h),3.80(s,3h).
169、13cnmr(101mhz,dmso)δ166.0,160.8,158.1,152.1,147.8,146.1,124.2,120.5,115.8,112.9,91.8,82.1,74.7,70.9,64.7,56.0.
170、hrms(esi,m/z)calcd.for c16h18n4o8na+:417.1017,found:417.1012.
171、制备实施例23
172、取代基r为丙磺舒骨架,制备实施方法和条件同制备实施例1;
173、(regioselective ratio);
174、1h nmr(400mhz,dmso)δ8.88(s,1h),8.21(d,j=8.29hz,2h),7.97(d,j=8.20hz,2h),7.92(s,1h),7.73(s,1h),5.97(d,j=2.77hz,1h),5.73(d,j=5.04hz,1h),5.47(d,j=5.91hz,1h),4.50(td,j=14.07,13.05,5.24hz,3h),4.43–4.39(m,1h),4.27(dt,j=6.54,3.79hz,1h),3.05(dd,j=8.72,6.32hz,4h),1.49–1.43(m,4h),0.80(t,j=7.34hz,6h).
175、13c nmr(101mhz,dmso)δ165.1,160.9,158.3,146.4,144.1,133.2,130.9,127.8,91.5,81.7,74.7,70.6,65.0,50.0,22.0,11.4.
176、hrms(esi,m/z)calcd.for c21h29n5o8sna+:534.1629,found:534.1623.
177、制备实施例24
178、取代基r为托灭酸骨架,制备实施方法和条件同制备实施例1;
179、
180、ratio);
181、1h nmr(400mhz,dmso)δ9.21(s,1h),8.86(s,1h),7.90(dd,j=8.07,1.69hz,1h),7.86–7.81(m,1h),7.66(s,1h),7.41–7.35(m,1h),7.31–7.21(m,3h),6.85–6.75(m,2h),5.96(d,j=2.67hz,1h),5.73(d,j=5.07hz,1h),5.45(d,j=6.00hz,1h),4.57–4.47(m,2h),4.45–4.37(m,2h),4.32–4.26(m,1h),2.23(s,3h).
182、13c nmr(101mhz,dmso)δ168.1,160.8,158.2,147.9,146.2,140.8,135.2,134.9,132.0,130.8,128.1,125.8,123.2,118.2,114.2,111.6,91.7,81.8,74.7,70.8,64.7,15.2.
183、hrms(esi,m/z)calcd.for c22h22cln5o6na+:510.1151,found:510.1146.
184、制备实施例25
185、n-杂化取代基为腺苷,制备实施方法和条件同制备实施例1;
186、ratio);
187、1h nmr(400mhz,dmso)δ8.29(s,1h),8.11(s,1h),7.94(dd,j=8.06,1.41hz,2h),7.67(t,j=7.55hz,1h),7.52(t,j=7.64hz,2h),7.30(s,2h),5.94(d,j=4.70hz,1h),5.59(d,j=5.67hz,1h),5.42(d,j=5.53hz,1h),4.81–4.74(m,1h),4.60(dd,j=12.07,3.70hz,1h),4.45(dt,j=10.09,5.23hz,2h),4.26–4.20(m,1h).
188、13c nmr(101mhz,dmso)δ166.1,156.6,153.1,149.8,140.4,133.9,129.9,129.7,129.2,119.7,88.6,81.9,73.3,70.6,64.8.
189、hrms(esi,m/z)calcd.for c17h17n5o5h+:372.1302,found:372.1302.
190、制备实施例26
191、n-杂化取代基为8-溴腺苷,制备实施方法和条件同制备实施例1;
192、
193、white solid,58%yield,26.1mg;>20:1(regioselective ratio);
194、1h nmr(400mhz,dmso)δ8.03(s,1h),7.86(dd,j=8.26,1.40hz,2h),7.67–7.61(m,1h),7.52–7.44(m,4h),5.85(d,j=4.50hz,1h),5.59(d,j=5.59hz,1h),5.43(d,j=5.71hz,1h),5.26(q,j=5.22hz,1h),4.66–4.58(m,2h),4.43(dd,j=12.08,5.02hz,1h),4.21(td,j=5.05,3.48hz,1h).
195、13c nmr(101mhz,dmso)δ166.0,155.5,153.2,150.7,133.8,129.9,129.6,129.1,127.7,120.0,91.1,82.1,71.3,70.3,64.2.
196、hrms(esi,m/z)calcd.for c17h16brn5o5h+:450.0408,found:450.0399.
197、制备实施例27
198、n-杂化取代基为鸟苷,制备实施方法和条件同制备实施例1;
199、
200、white solid,67%yield,25.8mg;15:1(regioselective ratio);
201、1h nmr(400mhz,dmso)δ10.69(s,1h),7.99–7.94(m,2h),7.85(s,1h),7.68(t,j=7.35hz,1h),7.54(t,j=7.67hz,2h),6.52(s,2h),5.75(d,j=5.10hz,1h),5.59(d,j=5.75hz,1h),5.36(d,j=5.41hz,1h),4.60–4.50(m,2h),4.44(dd,j=11.95,5.95hz,1h),4.33–4.28(m,1h),4.20–4.14(m,1h).
202、13c nmr(101mhz,dmso)δ166.1,157.2,154.2,151.8,135.9,134.0,129.9,129.7,129.3,117.3,87.4,81.8,73.6,70.8,65.0.
203、hrms(esi,m/z)calcd.for c17h17n5o6na+:410.1071,found:410.1063.
204、制备实施例28
205、n-杂化取代基为胞苷,制备实施方法和条件同制备实施例1;
206、
207、white solid,54%yield,18.7mg;19:1(regioselective ratio);
208、1h nmr(400mhz,dmso)δ8.02–7.97(m,2h),7.72–7.67(m,1h),7.60–7.54(m,3h),7.20(d,j=26.93hz,2h),5.79(d,j=3.44hz,1h),5.62(d,j=7.38hz,1h),5.44(d,j=5.00hz,1h),5.26(d,j=5.69hz,1h),4.58(dd,j=12.15,3.07hz,1h),4.44(dd,j=12.15,5.32hz,1h),4.12(td,j=5.67,3.07hz,1h),4.09–4.01(m,2h).
209、13c nmr(101mhz,dmso)δ166.1,166.0,155.5,141.7,134.0,129.9,129.7,129.3,94.6,90.8,80.9,74.0,70.1,64.7.
210、hrms(esi,m/z)calcd.for c16h17n3o6na+:370.1009,found:370.1005.
211、制备实施例29
212、n-杂化取代基为苯甲酰胞苷,制备实施方法和条件同制备实施例1;
213、
214、white solid,46%yield,20.6mg;>20:1(regioselective ratio);
215、1h nmr(400mhz,dmso)δ11.27(s,1h),8.18(d,j=7.53hz,1h),8.05–7.99(m,4h),7.71(t,j=7.48hz,1h),7.63(t,j=7.41hz,1h),7.57(t,j=7.72hz,2h),7.52(t,j=7.61hz,2h),7.29(d,j=7.50hz,1h),5.83(d,j=2.44hz,1h),5.66(d,j=4.64hz,1h),5.35(d,j=6.00hz,1h),4.65(dd,j=12.40,2.92hz,1h),4.55(dd,j=12.37,5.36hz,1h),4.28–4.22(m,1h),4.16–4.08(m,2h).
216、13c nmr(101mhz,dmso)δ167.8,166.1,163.7,154.9,151.8,145.6,134.1,133.6,133.2,129.9,129.7,129.3,128.9,96.7,91.9,81.2,74.3,69.7,64.5.
217、hrms(esi,m/z)calcd.for c23h21n3o7na+:474.1272,found:474.1266.
218、制备实施例30
219、n-杂化取代基为5-甲基尿苷,制备实施方法和条件同制备实施例1;
220、
221、white solid,51%yield,18.3mg;>20:1(regioselective ratio);
222、1h nmr(400mhz,dmso)δ11.36(s,1h),8.03–7.98(m,2h),7.69(t,j=7.42hz,1h),7.56(t,j=7.64hz,2h),7.37(s,1h),5.81(d,j=4.33hz,1h),5.48(d,j=4.61hz,1h),5.34(d,j=4.03hz,1h),4.59(dd,j=12.06,3.06hz,1h),4.43(dd,j=12.06,4.77hz,1h),4.18–4.11(m,3h),1.60(s,3h).
223、13c nmr(101mhz,dmso)δ166.1,164.1,151.2,136.5,134.1,129.9,129.7,129.4,110.3,88.7,81.5,73.1,70.3,64.8,12.3.
224、hrms(esi,m/z)calcd.for c17h18n2o7na+:385.1006,found:385.1002.
225、制备实施例31
226、取代基r为4-ch3-ph,制备实施方法和条件同制备实施例2;
227、
228、white solid,66%yield,23.7mg;4:1(regioselective ratio);
229、1h nmr(400mhz,dmso)δ8.93(s,1h),7.95(d,j=8.05hz,2h),7.87(s,1h),7.67(s,1h),7.39–7.36(m,2h),6.01(dd,j=9.31,5.55hz,2h),5.46(t,j=4.49hz,1h),5.12(t,j=5.64hz,1h),4.81(q,j=5.41hz,1h),4.31(q,j=4.45hz,1h),3.72–3.61(m,2h),2.41(s,3h).
230、13c nmr(101mhz,dmso)δ165.5,160.8,158.0,146.0,144.4,130.0,129.8,127.3,91.9,83.9,73.9,73.3,61.7,21.7.
231、hrms(esi,m/z)calcd.for c16h18n4o6na+:385.1118,found:385.1114.
232、制备实施例32
233、取代基r为4-och3-ph,制备实施方法和条件同制备实施例2;
234、
235、white solid,40%yield,15.3mg;4:1(regioselective ratio);
236、1h nmr(400mhz,dmso)δ8.93(s,1h),8.05–8.00(m,2h),7.87(s,1h),7.67(s,1h),7.10–7.07(m,2h),6.00(t,j=5.67hz,2h),5.44(t,j=4.56hz,1h),5.11(t,j=5.62hz,1h),4.80(q,j=5.43hz,1h),4.30(q,j=4.42hz,1h),3.86(s,3h),3.65(dt,j=14.21,5.50hz,2h).
237、13c nmr(101mhz,dmso)δ165.2,163.8,160.8,158.0,146.0,132.1,122.2,114.5,91.9,84.0,73.7,73.3,61.7,56.1.
238、hrms(esi,m/z)calcd.for c16h18n4o7na+:401.1068,found:401.1062.
239、制备实施例33
240、取代基r为4-f-ph,制备实施方法和条件同制备实施例2;
241、
242、white solid,69%yield,25.3mg;5:1(regioselective ratio);
243、1h nmr(400mhz,dmso)δ8.93(s,1h),8.16–8.11(m,2h),7.89–7.86(m,1h),7.68(s,1h),7.44–7.39(m,2h),6.05(d,j=6.10hz,1h),6.01(d,j=5.13hz,1h),5.48(dd,j=5.13,3.84hz,1h),5.13(t,j=5.65hz,1h),4.82(q,j=5.44hz,1h),4.33(q,j=4.41hz,1h),3.71–3.61(m,2h).
244、13c nmr(101mhz,dmso)δ167.0,164.6,159.4(d,j=278.9hz),149.6,145.9,132.9(d,j=9.5hz),126.6(d,j=2.8hz),116.3(d,j=22.3hz),91.9,83.9,74.3,73.4,61.7.
245、19f nmr(377mhz,dmso)δ-105.58.
246、hrms(esi,m/z)calcd.for c15h15fn4o6na+:389.0868,found:389.0864.
247、制备实施例34
248、取代基r为4-cl-ph,制备实施方法和条件同制备实施例2;
249、
250、white solid,61%yield,23.5mg;4:1(regioselective ratio);
251、1h nmr(400mhz,dmso)δ8.93(s,1h),8.06(d,j=8.58hz,2h),7.87(s,1h),7.67(s,1h),7.66–7.64(m,2h),6.06(d,j=6.04hz,1h),6.01(d,j=5.10hz,1h),5.49(dd,j=5.15,3.85hz,1h),5.12(t,j=5.67hz,1h),4.82(q,j=5.44hz,1h),4.33(q,j=4.44hz,1h),3.71–3.61(m,2h).
252、13c nmr(101mhz,dmso)δ164.7,160.8,158.0,146.0,139.0,131.8,129.4,128.8,91.8,83.8,74.4,73.3,61.6.
253、hrms(esi,m/z)calcd.for c15h15cln4o6na+:405.0572,found:405.0567.
254、制备实施例35
255、取代基r为4-br-ph,制备实施方法和条件同制备实施例2;
256、
257、white solid,60%yield,25.8mg;4:1(regioselective ratio);
258、1h nmr(400mhz,dmso)δ8.93(s,1h),8.00–7.97(m,2h),7.87(s,1h),7.81–7.78(m,2h),7.67(s,1h),6.06(d,j=6.01hz,1h),6.00(d,j=5.11hz,1h),5.48(dd,j=5.11,3.86hz,1h),5.13(t,j=5.65hz,1h),4.82(q,j=5.37hz,1h),4.33(q,j=4.43hz,1h),3.71–3.60(m,2h).
259、13c nmr(101mhz,dmso)δ164.9,160.8,158.0,146.0,132.4,131.9,129.2,128.1,91.8,83.8,74.4,73.3,61.6.
260、hrms(esi,m/z)calcd.for c15h15brn4o6na+:449.0067,found:449.0063.
261、制备实施例36
262、取代基r为3-ch3-ph,制备实施方法和条件同制备实施例2;
263、
264、white solid,71%yield,25.7mg;4:1(regioselective ratio);
265、1h nmr(400mhz,dmso)δ8.94(s,1h),7.88(s,1h),7.86(d,j=7.09hz,2h),7.68(s,1h),7.51(d,j=7.63hz,1h),7.46(dd,j=7.61,2.71hz,1h),6.03(dd,j=10.71,5.58hz,2h),5.48(t,j=4.49hz,1h),5.13(t,j=5.65hz,1h),4.82(q,j=5.43hz,1h),4.32(q,j=4.41hz,1h),3.72–3.62(m,2h),2.41(s,3h).
266、13c nmr(101mhz,dmso)δ165.6,160.8,158.0,146.0,138.6,134.6,130.3,129.9,129.1,127.2,91.8,83.9,74.0,73.3,61.7,21.3.
267、hrms(esi,m/z)calcd.for c16h18n4o6na+:385.1118,found:385.1112.
268、制备实施例37
269、取代基r为3-och3-ph,制备实施方法和条件同制备实施例2;
270、
271、white solid,70%yield,26.5mg;4:1(regioselective ratio);
272、1h nmr(400mhz,dmso)δ8.94(s,1h),7.88(s,1h),7.69–7.64(m,2h),7.54(d,j=1.12hz,1h),7.51–7.47(m,1h),7.28(dd,j=8.23,2.70hz,1h),6.04(d,j=6.09hz,1h),6.01(d,j=5.15hz,1h),5.48(dd,j=5.17,3.80hz,1h),5.12(t,j=5.66hz,1h),4.82(q,j=5.46hz,1h),4.32(q,j=4.42hz,1h),3.84(s,3h),3.71–3.62(m,2h).
273、13c nmr(101mhz,dmso)δ165.4,160.8,159.8,158.0,146.0,131.3,130.4,122.2,119.7,114.9,91.8,83.8,74.2,73.3,61.7,55.9.
274、hrms(esi,m/z)calcd.for c16h18n4o7na+:401.1068,found:401.1065.
275、制备实施例38
276、取代基r为3-f-ph,制备实施方法和条件同制备实施例2;
277、
278、white solid,75%yield,27.5mg;5:1(regioselective ratio);
279、1h nmr(400mhz,dmso)δ8.93(s,1h),7.92–7.89(m,1h),7.87(s,1h),7.83(dt,j=9.44,2.10hz,1h),7.68(s,1h),7.64–7.57(m,2h),6.06(d,j=6.16hz,1h),6.03(d,j=5.21hz,1h),5.50(dd,j=5.14,3.71hz,1h),5.13(t,j=5.66hz,1h),4.83(q,j=5.49hz,1h),4.34(q,j=4.39hz,1h),3.72–3.61(m,2h).
280、13c nmr(101mhz,dmso)δ164.5,162.5(d,j=244.88hz),160.8,158.0,146.0,132.3(d,j=7.5hz),131.6,126.2(d,j=2.6hz),121.1(d,j=20.9hz),116.5(d,j=22.8hz),91.7,83.7,74.7,73.3,61.6.
281、19f nmr(377mhz,dmso)δ-112.31.
282、hrms(esi,m/z)calcd.for c15h15fn4o6na+:389.0868,found:389.0863.
283、制备实施例39
284、取代基r为3-cl-ph,制备实施方法和条件同制备实施例2;
285、
286、white solid,66%yield,25.2mg;5:1(regioselective ratio);
287、1h nmr(400mhz,dmso)δ8.93(s,1h),8.06(q,j=2.90,2.38hz,1h),8.02(dt,j=7.72,1.36hz,1h),7.88(s,1h),7.81–7.78(m,1h),7.68(s,1h),7.62(t,j=7.89hz,1h),6.07(d,j=6.16hz,1h),6.03(d,j=5.20hz,1h),5.50(dd,j=5.17,3.73hz,1h),5.12(t,j=5.67hz,1h),4.83(q,j=5.52hz,1h),4.34(q,j=4.38hz,1h),3.72–3.63(m,2h).
288、13c nmr(101mhz,dmso)δ164.4,160.8,158.0,146.1,134.0,133.9,132.0,131.3,129.5,128.7,91.7,83.7,74.6,73.3,61.7.
289、hrms(esi,m/z)calcd.for c15h15cln4o6na+:405.0572,found:405.0570.
290、制备实施例40
291、取代基r为3-br-ph,制备实施方法和条件同制备实施例2;
292、
293、ratio);
294、1h nmr(400mhz,dmso)δ8.93(s,1h),8.19(t,j=1.94hz,1h),8.05(d,j=7.77hz,1h),7.94–7.87(m,2h),7.67(s,1h),7.55(t,j=7.91hz,1h),6.07(d,j=6.18hz,1h),6.03(d,j=5.22hz,1h),5.52–5.47(m,1h),5.12(t,j=5.66hz,1h),4.82(q,j=5.52hz,1h),4.34(q,j=4.33hz,1h),3.71–3.62(m,2h).
295、13c nmr(101mhz,dmso)δ164.3,160.8,158.0,146.1,136.8,132.3,132.2,131.5,129.0,122.3,91.7,83.7,74.6,73.3,61.7.
296、hrms(esi,m/z)calcd.for c15h15brn4o6na+:449.0067,found:449.0065.
297、制备实施例41
298、取代基r为2-ch3-ph,制备实施方法和条件同制备实施例2;
299、
300、white solid,46%yield,16.8mg;4:1(regioselective ratio);
301、1h nmr(400mhz,dmso)δ8.93(s,1h),7.95(d,j=7.43hz,1h),7.88(s,1h),7.67(s,1h),7.52(t,j=7.48hz,1h),7.36(d,j=7.39hz,2h),6.07(d,j=5.87hz,1h),5.98(d,j=4.94hz,1h),5.48(t,j=4.63hz,1h),5.13(t,j=5.69hz,1h),4.83(q,j=5.38hz,1h),4.32(d,j=4.41hz,1h),3.73–3.62(m,2h),2.57(s,3h).
302、13c nmr(101mhz,dmso)δ166.6,160.8,158.0,146.0,139.8,132.9,132.1,131.0,129.7,126.4,92.0,83.9,74.0,73.3,61.7,21.6.
303、hrms(esi,m/z)calcd.for c16h18n4o6na+:385.1118,found:385.1114.
304、制备实施例42
305、取代基r为2-och3-ph,制备实施方法和条件同制备实施例2;
306、
307、white solid,75%yield,28.4mg;3:1(regioselective ratio);
308、1h nmr(400mhz,dmso)δ8.93(s,1h),7.88(s,1h),7.80(dd,j=7.68,1.96hz,1h),7.67(s,1h),7.61–7.57(m,1h),7.20–7.17(m,1h),7.08–7.04(m,1h),6.02(d,j=5.88hz,1h),5.95(d,j=5.08hz,1h),5.44(dd,j=5.10,3.85hz,1h),5.13(t,j=5.64hz,1h),4.81(q,j=5.38hz,1h),4.27(q,j=4.41hz,1h),3.85(s,3h),3.71–3.61(m,2h).
309、13c nmr(101mhz,dmso)δ164.8,160.8,159.1,158.0,146.0,134.4,131.7,120.5,120.0,113.1,91.9,83.9,73.6,73.3,61.6,56.3.
310、hrms(esi,m/z)calcd.for c16h18n4o7na+:401.1068,found:401.1069.
311、制备实施例43
312、取代基r为2-f-ph,制备实施方法和条件同制备实施例2;
313、
314、white solid,62%yield,20.4mg;4:1(regioselective ratio);
315、1h nmr(400mhz,dmso)δ8.93(s,1h),8.00(t,j=7.59hz,1h),7.88(s,1h),7.72(d,j=6.79hz,1h),7.67(s,1h),7.41–7.36(m,2h),6.07(d,j=5.89hz,1h),5.96(d,j=5.10hz,1h),5.50(t,j=4.52hz,1h),5.13(t,j=5.68hz,1h),4.83(q,j=5.46hz,1h),3.72–3.62(m,2h).
316、13c nmr(101mhz,dmso)δ163.0,161.7(d,j=260.0hz),160.8,158.0,146.0,136.0(d,j=9.1hz),132.5,125.1(d,j=3.8hz),118.5(d,j=9.5hz),117.6(d,j=21.8hz),91.8,83.7,74.4,73.2,61.6.
317、19f nmr(377mhz,dmso)δ-110.27.
318、hrms(esi,m/z)calcd.for c15h15fn4o6na+:389.0868,found:389.0868.
319、制备实施例44
320、取代基r为2-cl-ph,制备实施方法和条件同制备实施例2;
321、
322、white solid,71%yield,27.3mg;7:1(regioselective ratio);
323、1h nmr(400mhz,dmso)δ8.93(s,1h),7.99–7.96(m,1h),7.88(s,1h),7.67(s,1h),7.64–7.61(m,2h),7.54–7.50(m,1h),6.11(d,j=5.89hz,1h),5.98(d,j=5.22hz,1h),5.52(dd,j=5.12,3.66hz,1h),5.14(t,j=5.67hz,1h),4.85(q,j=5.43hz,1h),4.32(q,j=4.45hz,1h),3.73–3.62(m,2h).
324、13cnmr(101mhz,dmso)δ164.4,160.8,158.0,146.1,134.1,132.8,132.2,131.4,129.9,127.9,91.8,83.7,74.7,73.2,61.6.
325、hrms(esi,m/z)calcd.for c15h15cln4o6na+:405.0572,found:405.0570.
326、制备实施例45
327、取代基r为2-br-ph,制备实施方法和条件同制备实施例2;
328、
329、white solid,77%yield,32.9mg;10:1(regioselective ratio);
330、1h nmr(400mhz,dmso)δ8.92(s,1h),7.96–7.92(m,1h),7.88(s,1h),7.80(dd,j=7.10,2.00hz,1h),7.67(s,1h),7.57–7.52(m,2h),6.11(d,j=5.90hz,1h),5.98(d,j=5.25hz,1h),5.51(dd,j=5.13,3.63hz,1h),5.14(t,j=5.62hz,1h),4.85(q,j=5.37hz,1h),4.33(q,j=4.47hz,1h),3.71–3.61(m,2h).
331、13cnmr(101mhz,dmso)δ165.1,160.8,158.0,146.0,134.6,134.0,132.1,132.0,128.3,121.1,91.9,83.8,74.8,73.3,61.7.
332、hrms(esi,m/z)calcd.for c15h15brn4o6na+:449.0067,found:449.0066.
333、制备实施例46
334、取代基r为2,6-cl-ph,制备实施方法和条件同制备实施例2;
335、
336、white solid,67%yield,28.0mg;7:1(regioselective ratio);
337、1h nmr(400mhz,dmso)δ8.91(s,1h),7.88(s,1h),7.68(s,1h),7.65–7.58(m,3h),6.09(d,j=6.25hz,1h),5.93(d,j=5.62hz,1h),5.55(dd,j=5.21,3.24hz,1h),5.16(t,j=5.66hz,1h),4.90(q,j=5.71hz,1h),4.29(q,j=4.34hz,1h),3.71(dt,j=12.11,5.04hz,1h),3.63(dt,j=11.70,5.38hz,1h).
338、13cnmr(101mhz,dmso)δ163.8,160.8,158.1,146.3,133.1,132.7,131.4,129.0,91.4,84.0,75.6,72.8,61.7.
339、hrms(esi,m/z)calcd.for c15h14cl2n4o6na+:439.0183,found:439.0178.
340、制备实施例47
341、取代基r为2,4,6-f-ph,制备实施方法和条件同制备实施例2;
342、
343、white solid,74%yield,23.8mg;4:1(regioselective ratio);
344、1h nmr(400mhz,dmso)δ8.92(s,1h),7.88(s,1h),7.67(s,1h),7.42(t,j=9.10hz,2h),6.10(d,j=5.88hz,1h),5.89(d,j=5.32hz,1h),5.54(dd,j=5.07,3.47hz,1h),5.15(t,j=5.69hz,1h),4.86(q,j=5.37hz,1h),4.25(q,j=4.46hz,1h),3.71–3.59(m,2h).
345、13cnmr(101mhz,dmso)δ160.8,159.6(d,j=23.2hz),158.0,146.1,133.1,102.7(d,j=3.8hz),102.4(d,j=3.6hz),102.2(d,j=3.5hz),91.8,83.8,75.3,73.0,61.6.
346、19f nmr(377mhz,dmso)δ-101.13(t,j=9.67hz),-106.01(d,j=9.99hz).
347、hrms(esi,m/z)calcd.for c15h13f3n4o6na+:425.0679,found:425.0677.
348、制备实施例48
349、取代基r为呋喃,制备实施方法和条件同制备实施例2;
350、
351、white solid,65%yield,22.0mg;10:1(regioselective ratio);
352、1h nmr(400mhz,dmso)δ8.92(s,1h),8.02(dd,j=1.74,0.84hz,1h),7.87(s,1h),7.67(s,1h),7.42(dd,j=3.52,0.91hz,1h),6.74(dd,j=3.52,1.75hz,1h),6.05(d,j=5.91hz,1h),5.95(d,j=5.11hz,1h),5.44(dd,j=5.11,3.79hz,1h),5.12(t,j=5.64hz,1h),4.80(q,j=5.39hz,1h),4.28(q,j=4.41hz,1h),3.70–3.59(m,2h).
353、13c nmr(101mhz,dmso)δ160.8,158.0,157.6,148.4,146.0,144.0,119.6,112.9,91.8,83.7,73.9,73.2,61.6.
354、hrms(esi,m/z)calcd.for c13h14fn4o7na+:361.0755,found:361.0757.
355、制备实施例49
356、取代基r为噻吩,制备实施方法和条件同制备实施例2;
357、
358、white solid,71%yield,25.2mg;5:1(regioselective ratio);
359、1h nmr(400mhz,dmso)δ8.93(s,1h),8.01(d,j=4.80hz,1h),7.91(dd,j=3.83,1.36hz,1h),7.87(s,1h),7.67(s,1h),7.26(dd,j=4.95,3.70hz,1h),6.06(d,j=5.88hz,1h),5.95(d,j=5.18hz,1h),5.47–5.41(m,1h),5.15(d,j=23.19hz,1h),4.80(t,j=5.19hz,1h),4.29(q,j=4.41hz,1h),3.71–3.59(m,2h).
360、13cnmr(101mhz,dmso)δ161.2,160.8,158.0,145.9,134.8,134.7,133.2,128.8,91.9,83.8,74.3,73.3,61.6.
361、hrms(esi,m/z)calcd.for c13h14n4o6sna+:377.0526,found:377.0524.
362、制备实施例50
363、取代基r为萘基,制备实施方法和条件同制备实施例2;
364、
365、white solid,61%yield,24.4mg;5:1(regioselective ratio);
366、1h nmr(400mhz,dmso)δ8.96(s,1h),8.74(s,1h),8.18(d,j=7.88hz,1h),8.09–8.04(m,3h),7.89(s,1h),7.71–7.64(m,3h),6.08(t,j=6.08hz,2h),5.56(t,j=4.49hz,1h),5.16(q,j=5.87,4.63hz,1h),4.87(q,j=5.40hz,1h),4.40(q,j=4.45hz,1h),3.76–3.65(m,2h).
367、13cnmr(101mhz,dmso)δ165.7,160.8,158.0,146.0,135.7,132.5,131.4,129.8,129.2,128.9,128.3,127.6,127.3,125.5,91.9,83.9,74.2,73.4,61.7.
368、hrms(esi,m/z)calcd.for c19h18n4o6na+:421.1118,found:421.1114.
369、制备实施例51
370、取代基r为烯基苯,制备实施方法和条件同制备实施例2;
371、
372、white solid,50%yield,18.9mg;4:1(regioselective ratio);
373、1h nmr(400mhz,dmso)δ8.92(s,1h),7.87(s,1h),7.76(q,j=3.55,3.11hz,3h),7.67(s,1h),7.47–7.44(m,3h),6.72(d,j=16.10hz,1h),6.01(d,j=6.02hz,1h),5.95(d,j=4.88hz,1h),5.37(t,j=4.65hz,1h),5.18–4.99(m,1h),4.76(t,j=5.13hz,1h),4.25(q,j=4.42hz,1h),3.69–3.58(m,2h).
374、13c nmr(101mhz,dmso)δ165.9,160.8,158.0,145.8,145.6,134.5,131.1,129.5,128.9,128.9,118.3,92.1,83.9,73.4,61.6.
375、hrms(esi,m/z)calcd.for c17h18n4o6na+:397.1118,found:397.1118.
376、制备实施例52
377、取代基r为柠檬醛骨架,制备实施方法和条件同制备实施例2;
378、
379、white solid,62%yield,24.4mg;3:1(regioselective ratio);
380、1h nmr(400mhz,dmso)δ8.91(s,1h),7.88(s,1h),7.68(s,1h),5.95(dd,j=5.93,4.60hz,1h),5.87(t,j=5.19hz,1h),5.76(dt,j=3.04,1.60hz,1h),5.25(t,j=4.70hz,1h),5.09(td,j=5.70,2.02hz,2h),4.70(q,j=5.29hz,1h),4.15(q,j=4.42hz,1h),3.63(dd,j=11.29,6.19hz,1h),3.58–3.52(m,1h),2.62–2.55(m,1h),2.19–2.15(m,2h),2.13(s,3h),1.92(s,1h),1.65(d,j=5.16hz,3h),1.59(s,3h).
381、13c nmr(101mhz,dmso)δ165.4,160.8,158.0,145.9,132.2,124.0,123.6,115.3,92.0,83.9,73.2,72.4,61.6,26.0,26.0,25.3,19.0,18.1.
382、hrms(esi,m/z)calcd.for c18h26n4o6na+:417.1745,found:417.1746.
383、制备实施例53
384、取代基r为香草醛骨架,制备实施方法和条件同制备实施例2;
385、
386、white solid,48%yield,18.8mg;2.4:1.0(regioselective ratio);
387、1h nmr(400mhz,dmso)δ10.02(s,1h),8.93(s,1h),7.87(s,1h),7.67(s,1h),7.58(dd,j=8.28,2.01hz,1h),7.51(d,j=1.99hz,1h),6.90(d,j=8.25hz,1h),6.04–5.95(m,2h),5.42(dd,j=5.20,3.93hz,1h),5.11(t,j=5.64hz,1h),4.82–4.75(m,1h),4.29(q,j=4.39hz,1h),3.84(s,3h),3.72–3.58(m,2h).
388、13c nmr(101mhz,dmso)δ165.4,160.8,158.0,152.3,147.9,145.9,124.4,120.7,115.6,113.4,91.9,84.0,73.6,73.4,61.7,56.2.
389、hrms(esi,m/z)calcd.for c16h18n4o8na+:417.1017,found:417.1016.
390、制备实施例54
391、取代基r为丙磺舒骨架,制备实施方法和条件同制备实施例2;
392、
393、white solid,62%yield,31.6mg;1.2:1.0(regioselective ratio);
394、1h nmr(400mhz,dmso)δ8.94(s,1h),8.24(d,j=8.27hz,2h),7.98(s,2h),7.89(s,1h),7.68(s,1h),6.02(d,j=5.03hz,1h),5.77–5.69(m,1h),5.53(t,j=4.52hz,1h),5.14(t,j=5.74hz,1h),4.84(q,j=5.24hz,1h),4.36(q,j=4.45hz,1h),3.74–3.63(m,2h),3.08–3.06(m,4h),1.49–1.46(m,4h),0.81(d,j=4.31hz,6h).
395、13c nmr(101mhz,dmso)δ164.5,160.8,158.0,146.0,144.2,133.4,130.9,127.6,91.8,83.7,74.8,73.3,61.6,50.0,22.0,11.4.
396、hrms(esi,m/z)calcd.for c21h29n5o8sna+:534.1629,found:534.1631.
397、制备实施例55
398、取代基r为托灭酸骨架,制备实施方法和条件同制备实施例2;
399、
400、e(58):
401、white solid,54%yield,26.5mg;1.0:1.0(regioselective ratio);
402、1h nmr(400mhz,dmso)δ9.16(s,1h),8.94(s,1h),8.06(dd,j=7.94,1.66hz,1h),7.88(s,1h),7.68(s,1h),7.41(dd,j=7.01,1.77hz,1h),7.29–7.27(m,3h),6.85–6.81(m,2h),6.13(d,j=5.88hz,1h),6.03(d,j=4.80hz,1h),5.51(t,j=4.66hz,1h),5.13(t,j=5.64hz,1h),4.84(q,j=5.21hz,1h),4.38–4.35(m,1h),3.73–3.64(m,2h),2.26(s,3h).
403、13c nmr(101mhz,dmso)δ167.4,160.8,158.0,148.0,146.0,140.7,135.3,135.0,132.3,130.9,128.1,125.8,123.3,117.9,114.3,111.6,92.0,83.8,73.3,70.8,56.5,19.0.
404、hrms(esi,m/z)calcd.for c22h22cln5o6na+:510.1151,found:510.1149.
405、制备实施例56
406、n-杂化取代基为5-甲基尿苷,制备实施方法和条件同制备实施例2;
407、
408、white solid,78%yield,28.2mg;3:1(regioselective ratio);
409、1h nmr(400mhz,dmso)δ11.38(s,1h),8.08–8.02(m,2h),7.78(d,j=1.50hz,1h),7.71–7.67(m,1h),7.57(t,j=7.75hz,2h),5.93(d,j=6.94hz,1h),5.81(d,j=6.13hz,1h),5.38(dd,j=5.55,2.67hz,1h),5.34(t,j=5.40hz,1h),4.41(q,j=6.23hz,1h),4.20(q,j=3.10hz,1h),3.72–3.66(m,2h),1.81(d,j=1.11hz,3h).
410、13c nmr(101mhz,dmso)δ165.6,164.1,151.4,136.6,133.9,130.0,129.9,129.2,110.3,87.7,83.0,74.0,72.1,61.6,12.6.
411、hrms(esi,m/z)calcd.for c17h18n2o7na+:385.1006,found:385.1000.
412、制备实施例57
413、n-杂化取代基为腺苷,制备实施方法和条件同制备实施例2;
414、
415、1h nmr(400mhz,dmso)δ8.41(s,1h),8.17(s,1h),8.11–8.07(m,2h),7.74–7.68(m,1h),7.59(t,j=7.70hz,2h),7.41(s,2h),6.03(d,j=7.22hz,1h),5.91(d,j=6.24hz,1h),5.76–5.71(m,1h),5.55(dd,j=5.29,1.98hz,1h),5.06–5.00(m,1h),4.32(q,j=2.99hz,1h),3.80–3.65(m,2h).
416、13c nmr(101mhz,dmso)δ165.6,156.8,156.7,153.0,140.4,134.0,130.2,129.9,129.2,120.1,88.3,84.1,74.7,72.4,62.1.
417、hrms(esi,m/z)calcd.for c17h17n5o5na+:394.1122,found:394.1117.
418、制备实施例58
419、n-杂化取代基为8-溴腺苷,制备实施方法和条件同制备实施例2;
420、
421、white solid,82%yield,36.9mg;8:1(regioselective ratio);
422、1h nmr(400mhz,dmso)δ8.17(s,1h),8.11–8.06(m,2h),7.71(t,j=7.46hz,1h),7.64(s,1h),7.59(t,j=7.65hz,3h),5.95(d,j=6.54hz,2h),5.76–5.72(m,1h),5.61(dd,j=5.67,2.37hz,1h),5.42(q,j=6.17hz,1h),4.36(q,j=3.38hz,1h),3.78(dt,j=12.34,4.06hz,1h),3.72–3.64(m,1h).
423、13c nmr(101mhz,dmso)δ165.5,155.7,153.1,150.4,134.0,130.2,129.9,129.2,127.4,120.1,91.0,84.3,74.4,70.3,62.3.
424、hrms(esi,m/z)calcd.for c17h16brn5o5na+:472.0227,found:472.0220.
425、制备实施例59
426、取代基r为2-甲基丙烯基,制备实施方法和条件同制备实施例1;
427、
428、white solid,41%yield,12.7mg;10:1(regioselective ratio);
429、1h nmr(400mhz,meod)δ7.71(d,j=7.50hz,1h),6.13(t,j=1.26hz,1h),5.87(d,j=7.52hz,1h),5.81(d,j=2.87hz,1h),5.69(q,j=1.62hz,1h),4.51(dd,j=12.42,2.89hz,1h),4.42(dd,j=12.45,4.65hz,1h),4.25–4.20(m,1h),4.16–4.09(m,2h),1.97(t,j=1.34hz,3h).
430、13c nmr(101mhz,meod)δ166.9,166.2,156.8,140.9,136.2,125.3,94.6,91.6,81.1,74.5,69.6,63.3,17.2.
431、hrms(esi,m/z)calcd.for c13h17n3o6na+:334.1010,found:334.1007.
432、制备实施例60
433、取代基r为异丙基;
434、
435、white solid,86%yield,28.3mg;10:1(regioselective ratio);
436、1h nmr(400mhz,dmso)δ10.01(s,1h),9.54(d,j=2.29hz,1h),6.83(d,j=8.25hz,1h),5.71(d,j=5.47hz,1h),5.58(dd,j=8.25,2.11hz,1h),5.37(d,j=5.75hz,1h),5.22(d,j=5.00hz,1h),4.25–4.11(m,2h),4.00(q,j=5.45hz,1h),3.95–3.88(m,2h),2.62–2.54(m,1h),1.11(s,3h),1.09(s,3h).
437、13c nmr(101mhz,meod)δ176.9,150.1,144.8,130.4,98.1,89.0,81.2,73.0,70.1,63.5,33.8,18.0,17.9.
438、hrms(esi,m/z)calcd.for c13h19n3o7na+:352.1115,found:352.1109.
439、制备实施例61
440、取代基r为2-甲基丙烯基,制备实施方法和条件同制备实施例1;
441、
442、white solid,50%yield,18.1mg;9:1(regioselective ratio);
443、1h nmr(400mhz,meod)δ7.84(s,1h),6.86(q,j=4.59hz,2h),6.05(t,j=1.33hz,1h),5.63–5.59(m,1h),4.88(s,1h),4.54(dd,j=12.01,2.88hz,1h),4.43–4.38(m,1h),4.35(dd,j=12.01,4.76hz,1h),4.20(dd,j=6.40,5.26hz,1h),1.89(t,j=1.31hz,3h).
444、13c nmr(101mhz,meod)δ166.9,155.8,146.9,136.0,125.3,124.2,116.6,116.2,110.7,101.1,81.9,80.0,74.1,70.5,62.9,17.1.
445、hrms(esi,m/z)calcd.for c16h17n5o5na+:382.1122,found:382.1119.
446、制备实施例62
447、取代基r为异丙基;
448、
449、white solid,91%yield,32.9mg;9:1(regioselective ratio);
450、1h nmr(400mhz,meod)δ7.86(s,1h),6.91–6.85(m,2h),4.87(d,j=6.15hz,1h),4.44–4.39(m,1h),4.38–4.34(m,1h),4.29(dd,j=11.61,4.78hz,1h),4.14(t,j=5.72hz,1h),2.58–2.50(m,1h),1.11(dd,j=6.98,4.10hz,6h).
451、13c nmr(101mhz,meod)δ146.9,144.5,124.3,116.6,116.2,110.7,101.1,98.7,82.0,80.0,74.2,70.6,62.9,33.7,17.9,17.8.
452、hrms(esi,m/z)calcd.for c16h19n5o5na+:384.1278,found:384.1279.
453、以下提供本发明合成的5’-o-酰化核苷类衍生物和3’-o-酰化核苷类衍生物共58个制备实施例抗病毒活性测试:
454、1、测试方法
455、根据文章报道(j.virol.methods 1997,69,137.;phytopathology1967,57,1285.),烟草花叶病毒(tmv)和马铃薯y病毒(pvy)从感染的普通烟草叶片中获得。采用半叶枯斑法,测试了选择性酰化核苷类衍生物对tmv和pvy的抗病毒活性。具体抑制率算法如下:
456、
457、1.1选择性酰化核苷类衍生物对tmv治疗活性
458、选长势一致的5-6叶期心叶烟,用毛笔蘸取病毒汁液磨擦接种于撒有金刚砂的叶片上,全叶接种病毒30分钟后,用清水冲洗。待叶片干后,在右半叶涂施药剂,左半叶涂施对应剂量的溶剂作对照。随后在光照培养箱中培养,1.5-2.0天后观察并记录产生枯斑的数目。
459、1.2选择性酰化核苷类衍生物对tmv保护活性
460、选长势一致的5-6叶期心叶烟,用毛笔轻轻在右半叶涂施药剂,左半叶涂施对应剂量的溶剂作对照。在光照培养箱中培养24小时后,用毛笔蘸病毒汁液人工摩擦接种于撒有金刚砂的叶片上,接种病毒30分钟后用清水冲洗叶片。并于光照培养箱中培养,1.5-2.0天后观察并记录产生枯斑的数目。
461、1.3选择性酰化核苷类衍生物对pvy治疗活性
462、选长势一致的5-6叶期苋色藜,用毛笔蘸取病毒汁液磨擦接种于撒有金刚砂的叶片上,全叶接种病毒60分钟后,用清水冲洗。待叶片干后,在右半叶涂施药剂,左半叶涂施对应剂量的溶剂作对照。随后在光照培养箱中培养,3-4天后观察并记录产生枯斑的数目。
463、1.4选择性酰化核苷类衍生物对pvy保护活性
464、选长势一致的5-6叶期苋色藜,用毛笔轻轻在右半叶涂施药剂,左半叶涂施对应剂量的溶剂作对照。在光照培养箱中培养24小时后,用毛笔蘸病毒汁液人工摩擦接种于撒有金刚砂的叶片上,接种病毒60分钟后用清水冲洗叶片。并于光照培养箱中培养,3-4天后观察并记录产生枯斑的数目。
465、2、抗植物病毒的生物活性测试结果
466、表1实施例1-58制备的单酯化核苷类化合物的抗tmv活性
467、
468、
469、通过半叶枯斑法,在浓度为500μg/ml的条件下,以商品药病毒唑和宁南霉素作为对照药,测试了单酯化核苷类化合物的抗tmv活性,实验结果见表1。生物活性测试结果表明化合物9、27、28、29和46表现出了优于对照药宁南霉素的抗tmv病毒活性,可作为潜在的抑植物病毒药物,具有较好应用前景。
470、表2实施例1-58制备的单酯化核苷类化合物的抗pvy活性
471、
472、
473、通过半叶枯斑法,在浓度为500μg/ml的条件下,以商品药病毒唑和宁南霉素作为对照药,测试了单酯化核苷类化合物的抗pvy活性,实验结果见表2。生物活性测试结果表明化合物9、29、42和49表现出了优于对照药宁南霉素的抗pvy病毒活性,可作为潜在的抑植物病毒药物,具有较好应用前景。
474、综合如上所述,仅是本发明的较佳实施例而已,并非对本发明作任何形式上的限制,任何未脱离本发明技术方案内容,依据本发明的技术实质对以上实施例所作的任何简单修改、等同变化与修饰,均仍属于本发明技术方案的范围内。
- 还没有人留言评论。精彩留言会获得点赞!