四环三萜衍生物及其药物组合物和应用的制作方法
本发明属于药物学领域,具体地涉及一种四环三萜衍生物及其药物组合物和应用。
背景技术:
肿瘤作为人类健康的一大威胁一直受到广泛的关注,寻找新的抗肿瘤药物是全世界药物化学家研究的重点和热点。从天然产物(naturalproducts)中寻找新的抗肿瘤药物是抗肿瘤药物发现的主要途径之一。三萜是一类具有30个碳原子构成基本碳架的结构独特的化合物,广泛存在于自然界,已经被分离、鉴定的化合物超过了20000种,具有抗炎、抗氧化、抗增殖、抗病毒等多种生物活性。
synthesisandanti-tumorevaluationofnovel25-hydroxyprotopanaxadiolanalogsincorporatingnaturalaminoacids和novelginsenosides25-oh-ppdand25-och3-ppdasexperimentaltherapyforpancreaticcancer:anticanceractivityandmechanismsofaction研究表明某些人参皂苷及其苷元具有显著的抗肿瘤作用。因此,有关低极性皂苷、苷元或二者的衍生物的制备与抗肿瘤活性的研究非常活跃。
dammarane-typetriterpenoidsfromgentianellaazurea,journalofnaturalproducts和bioactivedammaranetriterpenoidsfromthebarkofdrypetesacuminatafrompaluma,northqueensland,australia构效关系研究结果表明,母核结构的影响:原人参二醇型>原人参三醇型;糖基的影响:抗肿瘤活性研究表明,低糖链的皂苷及苷元具有更强的抗肿瘤作用。表现为:皂苷元>单糖苷>二糖苷>三糖苷>四糖苷。羟基的影响:在c-25位引入-oh或-ch3可以增强皂苷的活性。人参皂苷元ad-1,ad-2,ppd、pd、f11等能抑制人肿瘤细胞(人乳腺癌、人小细胞肺癌、人胃癌、人结肠癌、人神经胶质癌、人黑色素瘤、人宫颈癌、人肝癌、早幼粒白血病、肉瘤s-180、肝癌腹水型、小鼠宫颈癌-14及艾氏腹水癌等)生长与增殖,诱导肿瘤细胞分化、凋亡,抑制肿瘤新生血管生成、抑制肿瘤的浸润和转移、增强机体免疫力和降低化疗药物毒副作用等抗肿瘤活性。
sulfamicandsuccinicacidderivativesof25-oh-ppdandtheiractivitiestomcf-7,a-549,hct-116,andbgc-823celllines,sulfamicandsuccinicacidderivativesof25-oh-ppdandtheiractivitiestomcf-7,a-549,hct-116,andbgc-823celllines中表明人参皂苷元具有显著的体外抑制肿瘤细胞增殖活性,课题组前期对ad-1,ad-2,pd,ppd等结构修饰位点主要是3位和12位的羟基,涉及的反应主要为同链状小分子或芳香衍生物的酯化反应,获得了一系列活性增强的化合物。但ad-1,ad-2,pd,ppd等的抗肿瘤活性的结构修饰工作仍然有较大的探索空间。在此结构修饰的基础上,需要研发一种能够更加高效、低毒的新化合物。
技术实现要素:
发明目的:
本发明的目的在于针对现有技术的不足,提供一种四环三萜衍生物或其药用盐,该衍生物或其药用盐不仅具有显著增强的抑制肿瘤细胞增殖活性,而且安全低毒。
技术方案:
四环三萜衍生物,四环三萜衍生物的结构通式(ⅰ)为:
式(ⅰ)中,虚线部分表示为单键或双键;
r1为h,未被取代或为选自卤素、硝基、氰基、氨基、三氟甲基、苄氧基、氨基、二乙氨基、c1-8烷基、c1-8烷氧基中的一个或多个取代基所取代的苯乙烯基;
r2为h,未被取代或为选自卤素、硝基、氰基、氨基、苯基、c1-8烷基、c1-8烷氧基中的一个或多个取代基所取代的吡嗪羰基、吡啶羰基、吡咯羰基、哌啶羰基,
r3为h,未被取代或为选自卤素、硝基、氰基、氨基、苯基、c1-8烷基、c1-8烷氧基中的一个或多个取代基所取代的吡嗪羰基、吡啶羰基、吡咯羰基、哌啶羰基,
r4为
其中r5选自h、glc(6→1)ara(f)、glc、glc(6→1)glc、glc(6→1)ara(p)。
进一步的,通式(ⅰ)中r1,r2、r3为单取代,双取代或同时取代;且r1,r2、r3不可同时取h。
进一步的,通式(ⅰ)中r1为h、f、br、三氟甲基、二氟或甲氧基取代的苯乙烯基。
进一步的,通式(ⅰ)中r2和r3各自独立选自吡嗪羰基、吡啶羰基、吡咯羰基、哌啶羰基,其中烷基是具有0-20个碳原子的烷基。
进一步的,通式(ⅰ)中r2和r3各自独立为h,cl、甲基或苯基取代的哌啶羰基。
四环三萜衍生物的药学上可接受的盐,包括钾盐、钠盐、铵盐、镁盐、钙盐或盐酸盐。
四环三萜衍生物或其药学上可接受的盐,所述的四环三萜类化合物及其衍生物选自:
一种药物组合物,该药物组合物的活性成分至少包括所述四环三萜衍生物或其药学上可接受的盐,以及一种或多种药学上可接受的载体或赋形剂。
四环三萜衍生物或其药学上可接受的盐作为制备治疗肿瘤药物中的应用。
所述药物以四环三萜类化合物及其衍生物作为活性成分与药学上可接受的载体制备获得。
优点及技术效果:
与现有技术中的肿瘤药物相比,本发明的化合物抗肿瘤活性效果更优且毒性降低。
附图说明
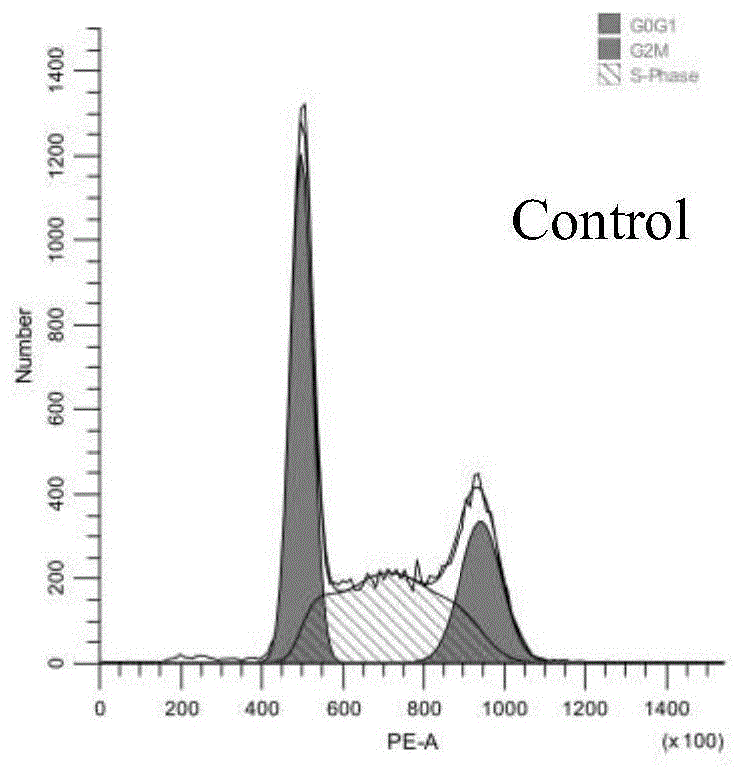
图1为空白组细胞周期流式图;
图2为化合物17(5μm)细胞周期流式图;
图3为化合物17(10μm)细胞周期流式图;
图4为化合物17不同浓度下细胞周期分布柱状图;
图5为空白组胞凋亡流式图;
图6为化合物17(10μm)细胞凋亡流式图;
图7为化合物17(15μm)细胞凋亡流式图;
图8为ad-2(10μm)细胞凋亡流式图;
图9为ad-2(15μm)细胞凋亡流式图;
图10为化合物17不同浓度下凋亡比例柱状图;
图11为ad-2不同浓度下凋亡比例柱状图。
具体实施方式
本发明原理是通过酯化反应,酮酯缩合,氧化反应,加成反应得到系列四环三萜衍生物。根据具体的目标化合物结构,设计相应的合成路线。举例如下:
本专利所列化合物,分别以20(r)-25-methoxyl-dammarane-3β,12β,20-triol(ad-1),20(r)-25-hydroxyl-dammarane-3β,12β,20-triol(ad-2)和dammar-20,25-epoxy-3β,12β-diol(pd)等为起始原料,参照以下路线所示方法,制备实施例中所列化合物。
合成路线举例如下所示:
路线1:
路线2:
路线3:
路线4:
路线5:
路线6:
路线7:
路线8:
路线9:
路线10:
路线11:
路线12:
以下将通过具体实施例进一步阐述本发明,但并不用于限制本发明的保护范围。
本发明的实施例中,1h-nmr与13c-nmr用varianmercury400核磁共振仪记录,化学位移以δ(ppm)表示;分离用硅胶未作特别说明时均为200-300目,洗脱液的配比均为体积比。
实施例1:
原料ad-2(4.56mg,10mmol)溶于无水二氯甲烷(50ml)中,搅拌下加入吡啶氯铬酸盐(4.08g,40mmol),室温下搅拌4h。反应结束,加入5%碳酸氢钠水溶液洗(80ml×3),有机层无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=5:1),得白色中间产物1共计3.59g,产率72%。
所得白色中间产物的核磁数据为:
1h-nmr(400mhz,cdcl3):δ3.51(1h,td,j=10.3,5.1hz,h-12),3.07(3h,s,–och3),1.05(9h,s,3×ch3),0.98(3h,s,ch3),0.94(3h,s,ch3),0.93(3h,s,ch3),0.88(3h,s,ch3),0.80(3h,s,ch3);13c-nmr(100mhz,cdcl3):δ39.86(c-1),34.21(c-2),217.90(c-3),47.51(c-4),55.45(c-5),19.80(c-6),34.21(c-7),39.79(c-8),49.48(c-9),36.94(c-10),31.58(c-11),70.81(c-12),49.26(c-13),51.75(c-14),31.13(c-15),26.44(c-16),49.89(c-17),16.07(c-18),16.07(c-19),74.92(c-20),21.98(c-21),43.16(c-22),17.42(c-23),40.61(c-24),74.92(c-25),25.10(c-26),25.10(c-27),26.85(c-28),21.17(c-29),17.19(c-30),48.80(–och3)。
实施例2:
中间体1(1.50g,3mmol)溶于20ml的无水四氢呋喃中,加入氢化钠(108mg,4.5mmol),随后加入对硝基苯甲醛(679.5mg,4.5mmol)。室温下反应4h。反应结束后,将反应液倾倒入40ml水中,加入乙酸乙酯(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=8:1),得白色产物2共计890mg,产率62%。
所得白色产物2的核磁数据为:
1hnmr(cdcl3,400mhz),δ8.26(d,2h,j=8hz),7.56(d,2h,j=8hz),7.48(s,1h),3.69(s,1h),1.88-1.78(m,3h),1.55-1.47(m,11h),1.25(s,12h),1.18(s,4h),1,14(s,3h),1.02(s,3h),0.94(s,3h),0.84(m,7h).13cnmr(cdcl3,100mhz),δ39.67(c-1),137.69(c-2),207.73(c-3),49.88(c-4),51.81(c-5),20.47(c-6),33.76(c-7),44.01(c-8),45.59(c-9),36.66(c-10),31.74(c-11),70.61(c-12),48.05(c-13),48.67(c-14),31.14(c-15),26.40(c-16),53.37(c-17),15.98(c-18),17.13(c-19),74.94(c-20),22.50(c-21),44.05(c-22),17.74(c-23),44.69(c-24),71.38(c-25),29.49(c-26),29.49(c-27),29.69(c-28),21.91(c-29),15.09(c-30),147.23(phe-c-no2),142.50(phe-c-c=c),134.78(-ch=ch-),130.82(2c,phe-c),123.91(2c,phe-c)。
实施例3
化合物2(1.2g,2mmol)溶于20ml甲醇中,加入nabh4(75.66mg,2mmol),室温下反应1h。反应完成后,将反应液倾倒入20ml水中,加入乙酸乙酯(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=10:1),得白色产物3共计1.05g,产率88%。
所得白色产物3的核磁数据为:
1hnmr(cdcl3,400mhz),δ8.18(d,2h,j=8hz),7.36(d,2h,j=8hz),6.80(s,1h),3.91(s,1h),3.63(s,1h),1.59-1.47(m,13h),1.26(s,5h),1.24(s,8h),1.16(s,4h),1.14(s,3h),0.95(s,5h),0.92(s,3h),0.78(s,3h),0.73(s,3h).13cnmr(cdcl3,100mhz),δ40.01(c-1),146.57(c-2),80.92(c-3),49.40(c-4),56.34(c-5),17.76(c-6),34.57(c-7),42.00(c-8),44.05(c-9),40.62(c-10),31.28(c-11),70.93(c-12),48.43(c-13),49.80(c-14),31.07(c-15),26.32(c-16),51.66(c-17),16.37(c-18),17.26(c-19),74.82(c-20),21.89(c-21),42.35(c-22),18.46(c-23),42.95(c-24),71.28(c-25),29.48(c-26),29.20(c-27),28.60(c-28),15.55(c-29),15.55(c-30),145.25(phe-c-no2),142.02(phe-c),129.55(2c,phe-c),123.68(2c,phe-c),121.64(-ch=ch-)。
实施例4
原料ad-1(4.91mg,10mmol)溶于无水二氯甲烷(50ml)中,搅拌下加入吡啶氯铬酸盐(4.08g,40mmol),室温下搅拌4h。反应结束,加入5%碳酸氢钠水溶液洗(80ml×3),有机层无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=5:1),得白色中间产物4共计3.59g,产率72%。
所得白色中间产物4的核磁数据为:
1h-nmr(400mhz,pyridine-d5):δ3.93(1h,td,j=8.9,4.9hz,h-12),1.44(9h,s,3×ch3),1.16(3h,s,ch3),1.07(3h,s,ch3),1.05(3h,s,ch3),0.93(3h,s,ch3),0.91(3h,s,ch3);13c-nmr(100mhz,pyridine-d5):δ40.12(c-1),34.66(c-2),216.76(c-3),47.76(c-4),55.61(c-5),19.13(c-6),34.81(c-7),40.28(c-8),50.07(c-9),37.29(c-10),32.92(c-11),71.06(c-12),49.84(c-13),52.13(c-14),31.80(c-15),27.04(c-16),51.13(c-17),15.90(c-18),16.44(c-19),73.77(c-20),23.21(c-21),44.47(c-22),20.30(c-23),46.01(c-24),70.09(c-25),30.63(c-26),30.34(c-27),27.19(c-28),21.56(c-29),17.64(c-30)。
实施例5
实施例5的中间体4(1.67g,3mmol)溶于20ml的无水四氢呋喃中,加入氢化钠(108mg,4.5mmol),随后加入对硝基苯甲醛(679.5mg,4.5mmol)。室温下反应4h。反应结束后,将反应液倾倒入40ml水中,加入乙酸乙酯(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=8:1),得白色产物5共计860mg,产率60%。
所得白色中间产物5的核磁数据为:
1hnmr(cdcl3,400mhz),δ7.12(dd,j1=8.4hz,j2=11.2hz),6.80(dd,j1=8hz,j2=12hz),6.56(s,1h),3.89(s,1h),3.56(m,1h),1.87-1.80(m,1h),1.69(m,3h),1.46(m,9h),1.22(s,8h),1.14(s,4h),1.11(s,3h),0.94(s,5h),0.90(s,7h),0.78(s,3h),0.69(s,3h).13cnmr(cdcl3,100mhz),δ39.96(c-1),142.92(c-2),80.91(c-3),49.37(c-4),56.34(c-5),17.63(c-6),34.59(c-7),41.67(c-8),44.06(c-9),40.13(c-10),31.25(c-11),70.61(c-12),48.43(c-13),49.79(c-14),31.03(c-15),51.61(c-17),15.98(c-18),17.16(c-19),74.47(c-20),21.88(c-21),42.69(c-22),18.46(c-23),42.98(c-24),71.10(c-25),29.40(c-26),28.50(c-27),29.70(c-28),15.44(c-29),15.52(c-30),162.90(phe-c-f),158.81(phe-c-f),131.27(phe-c),121.68(-c=c-),115.22(phe-c),110.90(phe-c),103.77(phe-c)。
实施例6
所得白色产物6的核磁数据为:
化合物5(1.17g,2mmol)溶于20ml甲醇中,加入nabh4(75.66mg,2mmol),室温下反应1h。反应完成后,将反应液倾倒入20ml水中,加入乙酸乙酯(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=10:1),得白色产物6共计1.01g,产率87%。
所得白色产物6的核磁数据为:
1hnmr(cdcl3,400mhz),δ7.16(dd,j1=5.6hz,j2=8.4hz),6.97(t,2h,j=8.4hz),6.67(s,1h),3.85(s,1h),3.58(s,1h),1.87-1.79(m,4h),1.60-1.39(m,13h),1.22(s,8h),1.13(s,3h),1.11(s,3h),1.07-1.03(m,3h),0.94(s,3h),0.89(s,3h),0.76(s,3h),0.73(s,3h).13cnmr(cdcl3,100mhz),δ39.95(c-1),140.32(c-2),80.96(c-3),49.32(c-4),56.42(c-5),18.42(c-6),34.61(c-7),41.65(c-8),44.05(c-9),40.23(c-10),31.29(c-11),70.68(c-12),48.39(c-13),49.79(c-14),31.04(c-15),26.30(c-16),51.63(c-17),16.26(c-18),17.19(c-19),74.39(c-20),21.92(c-21),42.07(c-22),17.64(c-23),42.96(c-24),71.11(c-25),29.29(c-26),28.54(c-27),29.59(c-28),15.50(c-29),15.53(c-30).162.46(phe-c-f),133.88(phe-c),130.32(phe-c),130.24(phe-c),121.84(phe-c),115.19(phe-c),114.97(phe-c)。
实施例7
原料pd(4.71mg,10mmol)溶于无水二氯甲烷(50ml)中,搅拌下加入吡啶氯铬酸盐(4.08g,40mmol),室温下搅拌4h。反应结束,加入5%碳酸氢钠水溶液洗(80ml×3),有机层无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=5:1),得白色中间产物7共计4.01g,产率84%。
所得白色中间产物7的核磁数据为:
1h-nmr(400mhz,cdcl3):δ3.55(1h,td,j=10.3,5.0hz,h-12),1.27(3h,s,ch3),1.22(3h,s,ch3),1.19(3h,s,ch3),1.08(3h,s,ch3),1.04(3h,s,ch3),1.02(3h,s,ch3),0.98(3h,s,ch3),0.89(3h,s,ch3);13c-nmr(100mhz,cdcl3):δ39.80(c-1),34.28(c-2),218.24(c-3),47.51(c-4),55.44(c-5),19.53(c-6),34.32(c-7),39.83(c-8),49.36(c-9),36.93(c-10),31.02(c-11),69.97(c-12),49.36(c-13),51.34(c-14),31.26(c-15),25.26(c-16),54.78(c-17),15.47(c-18),16.07(c-19),77.16(c-20),19.80(c-21),35.84(c-22),16.38(c-23),36.55(c-24),73.33(c-25),33.14(c-26),27.24(c-27),26.80(c-28),21.15(c-29),17.09(c-30)。
实施例8
中间体7(1.41g,3mmol)溶于20ml的无水四氢呋喃中,加入氢化钠(108mg,4.5mmol),随后加入2,4二氟苯甲醛(639.5mg,4.5mmol)。室温下反应4h。反应结束后,将反应液倾倒入40ml水中,加入乙酸乙酯(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=8:1),得白色产物8共计645mg,产率58%。
所得白色中间产物8的核磁数据为:
1hnmr(cdcl3,400mhz),δ7.12(dd,j1=8.4hz,j2=11.2hz),6.80(dd,j1=8hz,j2=12hz),6.56(s,1h),3.89(s,1h),3.56(m,1h),1.87-1.80(m,1h),1.69(m,3h),1.46(m,9h),1.22(s,8h),1.14(s,4h),1.11(s,3h),0.94(s,5h),0.90(s,7h),0.78(s,3h),0.69(s,3h).13cnmr(cdcl3,100mhz),δ39.96(c-1),142.92(c-2),80.91(c-3),49.37(c-4),56.34(c-5),17.63(c-6),34.59(c-7),41.67(c-8),44.06(c-9),40.13(c-10),31.25(c-11),70.61(c-12),48.43(c-13),49.79(c-14),31.03(c-15),51.61(c-17),15.98(c-18),17.16(c-19),74.47(c-20),21.88(c-21),42.69(c-22),18.46(c-23),42.98(c-24),71.10(c-25),29.40(c-26),28.50(c-27),29.70(c-28),15.44(c-29),15.52(c-30),162.90(phe-c-f),158.81(phe-c-f),131.27(phe-c),121.68(-c=c-),115.22(phe-c),110.90(phe-c),103.77(phe-c)。
实施例9
化合物8(1.17g,2mmol)溶于20ml甲醇中,加入nabh4(75.66mg,2mmol),室温下反应1h。反应完成后,将反应液倾倒入20ml水中,加入乙酸乙酯(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=10:1),得白色产物9共计1.02g,产率88%。
所得白色产物9的核磁数据为:
1hnmr(cdcl3,400mhz),δ7.44(s,1h),7.42(m,2h),7.10(t,2h,j=8hz),3.68(td,1h,j1=4hz,j2=8hz),1.91(m,2h),1.80(m,2h),1.58-1.43(m,12h),1.37-1.30(m,3h),1.23(s,8h),1.18(s,3h),1.16(s,3h),1.13(s,3h),1.02(s,4h),0.94(s,3h),0.84(s,3h).13cnmr(cdcl3,100mhz),δ39.53(c-1),136.22(c-2),207.95(c-3),49.89(c-4),51.68(c-5),20.39(c-6),33.69(c-7),43.00(c-8),45.24(c-9),36.39(c-10),31.76(c-11),70.52(c-12),48.03(c-13),48.63(c-14),31.01(c-15),26.32(c-16),53.14(c-17),15.82(c-18),17.01(c-19),74.47(c-20),22.31(c-21),44.06(c-22),17.62(c-23),44.61(c-24),71.10(c-25),29.52(c-26),29.41(c-27),29.70(c-28),21.84(c-29),14.96(c-30),163.80(phe-c-f),133.67(phe-c),132.03(-c=c-),132.23(phe-c),132.15(phe-c),115.4(phe-c),115.4(phe-c)。
实施例10
1-boc-4-哌啶甲酸(458.54mg,2mmol)溶于20ml的无水二氯甲烷中,依次加入1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(edc,382mg,2mmol)4-二甲氨基吡啶(dmap,12.2mg,0.01mmol),室温下磁力搅拌30min后,加入ad-2(478mg,2mmol)。室温下反应2h。反应结束后,加入二氯甲烷(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=5:1),得白色产物10共计224mg,产率51%。
所得白色产物10的核磁数据为:
1hnmr(cdcl3,400mhz),δ4.49(t,1h,j=8hz),4.02(d,2h,j=15hz,3’aand5’a),3.61(td,1h,j1=4hz,j2=12hz,12-h),2.84(t,3h,j=12hz),2.45(m,1h),2.07(m,1h),1.89(m,5h),1.75-1.62(m,7h),1.46(s,18h),1.23(s,12h),1.14(s,3h),0.99(s,3h),0.87(m,12h).13cnmr(cdcl3,100mhz),δ40.08(c-1),24.00(c-2),81.04(c-3),38.35(c-4),56.24(c-5),18.01(c-6),37.36(c-7),41.07(c-8),50.22(c-9),37.36(c-10),31.47(c-11),71.57(c-12),48.63(c-13),51.97(c-14),31.39(c-15),26.66(c-16),50.29(c-17),16.57(c-18),16.93(c-19),79.94(c-20),22.10(c-21),41.87(c-22),18.47(c-23),43.13(c-24),71.30(c-25),30.04(c-26),29.72(c-27),28.57(c-28),15.97(c-29),17.45(c-30).174.55(-coo-),155.08(-coo-nh-),79.99(boc-ch-o-),44.42(-ch2-nh-),43.55(-ch2-nh-),38.91(-ch-coo-),28.78(3c,boc-ch3),28.37(-ch2-),28.19(-ch2-)。
实施例11
化合物10(689.1mg,1mmol)溶于15ml的干燥甲苯中,加入300-400目硅胶(861.25mg,1.25mmol),120℃加热回流12h。反应结束后,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=4:1),得白色产物11共计321mg,产率52%。
所得白色产物11的核磁数据为:
1hnmr(pyridine-d6,400mhz),δ4.67(t,1h,3-h),3.69(m,1h),3.32(m,2h,3’aand5’a),2.84(m,1h),2.43(m,3h),1.75(m,4h),1.59(m,5h),1.44(s,14h),1.27(s,14h),1.05(s,3h),0.95(s,3h),0.89(s,3h)0.86(s,3h).13cnmr(pyridine-d6,100mhz),δ39.14(c-1),23.66(c-2),80.86(c-3),37.92(c-4),56.03(c-5),18.19(c-6),34.79(c-7),39.80(c-8),50.00(c-9),36.93(c-10),31.95(c-11),70.52(c-12),49.04(c-13),51.52(c-14),31.21(c-15),26.44(c-16),50.55(c-17),16.06(c-18),16.56(c-19),73.12(c-20),22.59(c-21),42.70(c-22),18.49(c-23),43.84(c-24),69.57(c-25),29.96(c-26),29.69(c-27),27.90(c-28),15.61(c-29),17.15(c-30),172.92(-coo-),45.63(-ch2-nh-),45.37(-ch2-nh-),38.30(-ch-coo-),25.37(-ch2-),25.21(-ch2-)。
实施例12
2-吡嗪甲酸(248.04mg,2mmol)溶于20ml的无水二氯甲烷中,依次加入1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(edc,382mg,2mmol),4-二甲氨基吡啶(dmap,12.2mg,0.01mmol),室温下磁力搅拌30min后,加入ad-1(478mg,2mmol)。室温下反应2h。反应结束后,加入二氯甲烷(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=5:1),得白色产物12共计267mg,产率55%。
所得白色产物12的核磁数据为:
1hnmr(pyridine-d6,400mhz),δ4.62(m,1h,3-h),3.62(t,1h,j=8hz),3.56(d,1h,j=8hz),3.39(t,1h,j=8hz),3.08(m,2h),2.68(s,1h),2.23(d,j=8hz),2.12(m,1h),1.93(td,1h,j1=8hz,j2=16hz),1.74(d,5h,j=4hz),1.42(s,20h),1.25(s,16h),1.02(s,4h),0.94(s,3h),0.83(m,8h),0.73(m,2h).13cnmr(pyridine-d6,100mhz),δ39.09(c-1),23.80(c-2),81.22(c-3),38.17(c-4),55.96(c-5),18.39(c-6),35.01(c-7),40.02(c-8),50.24(c-9),37.13(c-10),32.16(c-11),70.74(c-12),49.26(c-13),51.72(c-14),31.42(c-15),26.41(c-16),50.76(c-17),16.22(c-18),16.72(c-19),73.32(c-20),21.89(c-21),43.56(c-22),18.69(c-23),44.04(c-24),69.74(c-25),30.12(c-26),29.89(c-27),28.07(c-28),15.82(c-29),17.36(c-30),171.99(-coo-),45.57(-ch2-nh-),44.74(-nh-ch2-),38.52(-ch-coo-),26.65(-ch2-),22.78(-ch2-)。
实施例13
1-boc-3-哌啶甲酸(458.54mg,2mmol)溶于20ml的无水二氯甲烷中,依次加入1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(edc,382mg,2mmol)4-二甲氨基吡啶(dmap,12.2mg,0.01mmol),室温下磁力搅拌30min后,加入pd(942mg,2mmol)。室温下反应2h。反应结束后,加入二氯甲烷(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=5:1),得白色产物13共计531mg,产率53%。
所得白色产物13的核磁数据为:
1hnmr(pyridine-d6,400mhz),δ4.49(t,j=8hz),4.15(m,1h),3.93(m,1h),3.60(td,j1=4hz,j2=8hz),2.94(s,1h),2.79(m,1h),2.44(s,1h),2.06(m,2h),1.83(m,2h),1.61-1.75(m,7h),1.45(s,23h),1.24(m,9h),1.13(s,3h),0.99(s,3h),0.90-0.84(m,13h).13cnmr(cdcl3,100mhz),δ39.74(c-1),23.67(c-2),79.67(c-3),37.99(c-4),55.90(c-5),17.68(c-6),34.67(c-7),41.77(c-8),49.83(c-9),37.02(c-10),31.27(c-11),71.12(c-12),48.37(c-13),51.60(c-14),31.02(c-15),26.35(c-16),49.95(c-17),16.18(c-18),16.63(c-19),74.28(c-20),21.89(c-21),42.93(c-22),18.15(c-23),44.16(c-24),70.81(c-25),29.43(c-26),29.38(c-27),28.04(c-28),15.65(c-29),17.14(c-30),173.11(-coo-),154.71(-coo-nh-),80.77(boc-ch-o-),45.51(-ch2-nh-),45.15(-nh-ch2-),38.56(-ch-coo-),28.43((3c,boc-ch3),27.63(-ch2-),24.44(-ch2-)。
实施例14
化合物13(689.1mg,1mmol)溶于15ml的干燥甲苯中,加入300-400目硅胶(861.25mg,1.25mmol),120℃加热回流12h。反应结束后,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=4:1),得白色产物14共计321mg,产率52%。
所得白色产物14的核磁数据为:
1hnmr(pyridine-d6,400mhz),δ4.62(m,1h,3-h),3.62(t,1h,j=8hz),3.56(d,1h,j=8hz),3.39(t,1h,j=8hz),3.08(m,2h),2.68(s,1h),2.23(d,j=8hz),2.12(m,1h),1.93(td,1h,j1=8hz,j2=16hz),1.74(d,5h,j=4hz),1.42(s,20h),1.25(s,16h),1.02(s,4h),0.94(s,3h),0.83(m,8h),0.73(m,2h).13cnmr(pyridine-d6,100mhz),δ39.09(c-1),23.80(c-2),81.22(c-3),38.17(c-4),55.96(c-5),18.39(c-6),35.01(c-7),40.02(c-8),50.24(c-9),37.13(c-10),32.16(c-11),70.74(c-12),49.26(c-13),51.72(c-14),31.42(c-15),26.41(c-16),50.76(c-17),16.22(c-18),16.72(c-19),73.32(c-20),21.89(c-21),43.56(c-22),18.69(c-23),44.04(c-24),69.74(c-25),30.12(c-26),29.89(c-27),28.07(c-28),15.82(c-29),17.36(c-30),171.99(-coo-),45.57(-ch2-nh-),44.74(-nh-ch2-),38.52(-ch-coo-),26.65(-ch2-),22.78(-ch2-)。
实施例15
3-溴丙酸(305.94mg,2mmol)溶于20ml的无水二氯甲烷中,依次加入1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(edc,382mg,2mmol),4-二甲氨基吡啶(dmap,12.2mg,0.01mmol),室温下磁力搅拌30min后,加入ad-1(942mg,2mmol)。室温下反应2h。反应结束后,加入二氯甲烷(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=7:1),得白色产物15共计341mg,产率47%。
所得白色产物15的核磁数据为:
1hnmr(500mhz,cdcl3):δ=7.36-7.29(m,5h),5.24-5.23(m,1h),5.10(d,j=12.5hz,1h),4.98(d,j=12.5hz,1h),3.20(dd,j=11.0,4.5hz,1h),2.04-1.98(m,1h),1.89-1.76(m,3h),1.73-1.67(m,2h),1.64-1.55(m,4h),1.53-1.44(m,6h),1.37-1.26(m,6h),1.07(s,3h),1.05-1.02(m,2h),0.98(s,3h),0.93(d,j=6.0hz,3h),0.89(s,3h),0.85(d,j=6.5hz,3h),0.78(s,3h),0.64(s,3h)ppm.13cnmr(125mhz,cdcl3):δ=177.3,138.1,136.3,128.4(2c),128.1(2c),127.9,125.7,79.0,66.0,55.2,52.9,48.1,47.5,42.0,39.5,39.1,38.8,38.7,38.6,36.9,36.6,33.0,30.6,28.1,27.9,27.2,24.2,23.5,23.2,21.2,18.3,17.0(2c),15.6,15.4。
实施例16
化合物15(809.53mg,1mmol)溶于20ml的乙腈,加入三苯基膦(314mg,1.2mmol)。50℃加热回流12h。反应结束后,减压浓缩除去溶剂,硅胶柱层析(二氯甲烷:甲醇=30:1),得白色产物16共计214mg,产率32%。
所得白色产物16的核磁数据为:
1h-nmr(400mhz,cdcl3):δ3.18(3h,s,–och3),1.22(3h,s,ch3),1.15(6h,s,2×ch3),1.10(3h,s,ch3),1.06(3h,s,ch3),1.03(3h,s,ch3),1.00(3h,s,ch3),0.81(3h,s,ch3);13c-nmr(100mhz,cdcl3):δ39.21(c-1),33.34(c-2),216.90(c-3),47.53(c-4),55.30(c-5),19.80(c-6),33.92(c-7),39.14(c-8),52.70(c-9),37.24(c-10),40.19(c-11),214.21(c-12),56.72(c-13),54.03(c-14),30.97(c-15),25.23(c-16),44.06(c-17),15.63(c-18),15.63(c-19),73.65(c-20),21.98(c-21),43.22(c-22),17.70(c-23),40.56(c-24),74.88(c-25),25.13(c-26),25.17(c-27),26.68(c-28),21.21(c-29),17.48(c-30),49.25(–och3)。
实施例17
2-氯吡啶甲酸(458.54mg,2mmol)溶于20ml的无水二氯甲烷中,依次加入1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(edc,382mg,2mmol),4-二甲氨基吡啶(dmap,12.2mg,0.01mmol),室温下磁力搅拌30min后,加入pd(942mg,2mmol)。室温下反应2h。反应结束后,加入二氯甲烷(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=5:1),得白色产物17共计535mg,产率54%。
所得白色产物17的核磁数据为:
1h-nmr(600mhz,cdcl3):δ8.42(1h,d,j=1.8hz,n–ch),8.26(1h,d,j=2.4hz,n–ch),3.67(1h,td,j=10.4,5.0hz,h-12),3.10(1h,d,j=16.5hz,h-1),2.54(1h,d,j=15.1hz,h-1),1.31(3h,s,ch3),1.30(3h,s,ch3),1.24(3h,s,ch3),1.23(3h,s,ch3),1.14(3h,s,ch3),1.07(3h,s,ch3),0.94(3h,s,ch3),0.86(3h,s,ch3);13c-nmr(150mhz,cdcl3):δ48.91(c-1),150.60(c-2),159.78(c-3),39.72(c-4),53.58(c-5),20.23(c-6),34.09(c-7),39.70(c-8),48.51(c-9),36.78(c-10),31.97(c-11),70.71(c-12),48.60(c-13),51.85(c-14),31.17(c-15),26.51(c-16),50.41(c-17),15.29(c-18),16.3(c-19),74.14(c-20),21.92(c-21),43.11(c-22),18.00(c-23),44.52(c-24),71.12(c-25),29.56(c-26),29.53(c-27),31.70(c-28),24.15(c-29),17.21(c-30),141.56(n–ch),142.62(n–ch).
实施例18
3-溴丙酸(304.43mg,2mmol)溶于20ml的无水二氯甲烷中,依次加入1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(edc,382mg,2mmol),4-二甲氨基吡啶(dmap,12.2mg,0.01mmol),室温下磁力搅拌30min后,加入ad-1(982mg,2mmol)。室温下反应2h。反应结束后,加入二氯甲烷(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=5:1),得白色产物18共计530mg,产率53%。
所得白色产物18的核磁数据为:
1h-nmr(400mhz,pyridine-d5):δ4.05(1h,td,j=9.5,4.4hz,h-12),3.15(3h,s,–och3),1.62(3h,s,ch3),1.56(3h,s,ch3),1.42(3h,s,ch3),1.16(3h,s,ch3),1.15(9h,s,3×ch3),1.07(3h,s,ch3);13c-nmr(100mhz,pyridine-d5):δ43.57(c-1),174.81(c-2),182.80(c-3),47.50(c-4),49.19(c-5),18.45(c-6),34.93(c-7),40.31(c-8),42.60(c-9),42.43(c-10),31.86(c-11),71.23(c-12),49.72(c-13),52.70(c-14),31.80(c-15),27.15(c-16),51.11(c-17),16.08(c-18),20.91(c-19),73.40(c-20),23.65(c-21),44.12(c-22),19.60(c-23),41.51(c-24),74.91(c-25),25.52(c-26),25.52(c-27),28.14(c-28),25.34(c-29),17.87(c-30),49.30(–och3)。
实施例19
化合物18(724.53mg,1mmol)溶于20ml的乙腈,加入三苯基膦(314mg,1.2mmol)。50℃加热回流12h。反应结束后,减压浓缩除去溶剂,硅胶柱层析(二氯甲烷:甲醇=30:1),得白色产物19共计203mg,产率30%。
所得白色产物19的核磁数据为:
1h-nmr(400mhz,cdcl3):δ3.51(1h,td,j=10.3,5.1hz,h-12),3.07(3h,s,–och3),1.05(9h,s,3×ch3),0.98(3h,s,ch3),0.94(3h,s,ch3),0.93(3h,s,ch3),0.88(3h,s,ch3),0.80(3h,s,ch3);13c-nmr(100mhz,cdcl3):δ39.86(c-1),34.21(c-2),217.90(c-3),47.51(c-4),55.45(c-5),19.80(c-6),34.21(c-7),39.79(c-8),49.48(c-9),36.94(c-10),31.58(c-11),70.81(c-12),49.26(c-13),51.75(c-14),31.13(c-15),26.44(c-16),49.89(c-17),16.07(c-18),16.07(c-19),74.92(c-20),21.98(c-21),43.16(c-22),17.42(c-23),40.61(c-24),74.92(c-25),25.10(c-26),25.10(c-27),26.85(c-28),21.17(c-29),17.19(c-30),48.80(–och3)。
实施例20
2-吡嗪甲酸(458.54mg,2mmol)溶于20ml的无水二氯甲烷中,依次加入1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(edc,382mg,2mmol),4-二甲氨基吡啶(dmap,12.2mg,0.01mmol),室温下磁力搅拌30min后,加入化合物11(97976mg,2mmol)。室温下反应2h。反应结束后,加入二氯甲烷(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=5:1),得白色产物20共计595mg,产率57%。
所得白色产物20的核磁数据为:
1hnmr(400mhz,cdcl3):δ8.43(1h,d,j=1.8hz,n–ch),8.29(1h,d,j=2.3hz,n–ch),1.32(6h,s,2×ch3),1.25(3h,s,ch3),1.22(6h,s,2×ch3),1.01(3h,s,ch3),0.90(3h,s,ch3),0.85(3h,s,ch3);13c-nmr(100mhz,cdcl3):δ48.21(c-1),149.83(c-2),159.31(c-3),39.70(c-4),54.73(c-5),20.24(c-6),33.10(c-7),40.10(c-8),51.72(c-9),37.09(c-10),39.14(c-11),214.10(c-12),56.66(c-13),53.34(c-14),31.00(c-15),25.22(c-16),43.18(c-17),15.32(c-18),15.93(c-19),73.62(c-20),22.03(c-21),44.10(c-22),18.13(c-23),44.57(c-24),71.20(c-25),29.54(c-26),29.36(c-27),31.65(c-28),24.15(c-29),17.49(c-30),141.73(n–ch),142.72(n–ch)。
实施例21
2-吡嗪甲酸(248.04mg,2mmol)溶于20ml的无水二氯甲烷中,依次加入1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(edc,382mg,2mmol),4-二甲氨基吡啶(dmap,12.2mg,0.01mmol),室温下磁力搅拌30min后,加入compoundk(412mg,2mmol)。室温下反应2h。反应结束后,加入二氯甲烷(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=5:1),得白色产物21共计201mg,产率50%。
所得白色产物21的核磁数据为:
1hnmr(pyridine-d6,400mhz),δ4.62(m,1h,3-h),3.62(t,1h,j=8hz),3.56(d,1h,j=8hz),3.39(t,1h,j=8hz),3.08(m,2h),2.68(s,1h),2.23(d,j=8hz),2.12(m,1h),1.93(td,1h,j1=8hz,j2=16hz),1.74(d,5h,j=4hz),1.42(s,20h),1.25(s,16h),1.02(s,4h),0.94(s,3h),0.83(m,8h),0.73(m,2h).13cnmr(pyridine-d6,100mhz),δ39.09(c-1),23.80(c-2),81.22(c-3),38.17(c-4),55.96(c-5),18.39(c-6),35.01(c-7),40.02(c-8),50.24(c-9),37.13(c-10),32.16(c-11),70.74(c-12),49.26(c-13),51.72(c-14),31.42(c-15),26.41(c-16),50.76(c-17),16.22(c-18),16.72(c-19),73.32(c-20),21.89(c-21),43.56(c-22),18.69(c-23),44.04(c-24),69.74(c-25),30.12(c-26),29.89(c-27),28.07(c-28),15.82(c-29),17.36(c-30),171.99(-coo-),45.57(-ch2-nh-),44.74(-nh-ch2-),38.52(-ch-coo-),26.65(-ch2-),22.78(-ch2-)。
实施例22
1-boc-4-哌啶甲酸(442.31mg,2mmol)溶于20ml的无水二氯甲烷中,依次加入1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(edc,380mg,2mmol)4-二甲氨基吡啶(dmap,12.0mg,0.01mmol),室温下磁力搅拌30min后,加入ad-1(931mg,2mmol)。室温下反应2h。反应结束后,加入二氯甲烷(80ml×3)进行萃取,合并有机相,无水硫酸钠干燥,滤过,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=5:1),得白色产物22共计431mg,产率50%。
所得白色产物22的核磁数据为:
1hnmr(pyridine-d6,400mhz),δ4.49(t,j=8hz),4.15(m,1h),3.93(m,1h),3.60(td,j1=4hz,j2=8hz),2.94(s,1h),2.79(m,1h),2.44(s,1h),2.06(m,2h),1.83(m,2h),1.61-1.75(m,7h),1.45(s,23h),1.24(m,9h),1.13(s,3h),0.99(s,3h),0.90-0.84(m,13h).13cnmr(cdcl3,100mhz),δ39.74(c-1),23.67(c-2),79.67(c-3),37.99(c-4),55.90(c-5),17.68(c-6),34.67(c-7),41.77(c-8),49.83(c-9),37.02(c-10),31.27(c-11),71.12(c-12),48.37(c-13),51.60(c-14),31.02(c-15),26.35(c-16),49.95(c-17),16.18(c-18),16.63(c-19),74.28(c-20),21.89(c-21),42.93(c-22),18.15(c-23),44.16(c-24),70.81(c-25),29.43(c-26),29.38(c-27),28.04(c-28),15.65(c-29),17.14(c-30),173.11(-coo-),154.71(-coo-nh-),80.77(boc-ch-o-),45.51(-ch2-nh-),45.15(-nh-ch2-),38.56(-ch-coo-),28.43((3c,boc-ch3),27.63(-ch2-),24.44(-ch2-)。
实施例23
化合物22(607.1mg,1mmol)溶于15ml的干燥甲苯中,加入300-400目硅胶(845.25mg,1.25mmol),120℃加热回流12h。反应结束后,减压浓缩除去溶剂,硅胶柱层析(石油醚:丙酮=4:1),得白色产物23共计312mg,产率50%。
1hnmr(pyridine-d6,400mhz),δ4.62(m,1h,3-h),3.62(t,1h,j=8hz),3.56(d,1h,j=8hz),3.39(t,1h,j=8hz),3.08(m,2h),2.68(s,1h),2.23(d,j=8hz),2.12(m,1h),1.93(td,1h,j1=8hz,j2=16hz),1.74(d,5h,j=4hz),1.42(s,20h),1.25(s,16h),1.02(s,4h),0.94(s,3h),0.83(m,8h),0.73(m,2h).13cnmr(pyridine-d6,100mhz),δ39.09(c-1),23.80(c-2),81.22(c-3),38.17(c-4),55.96(c-5),18.39(c-6),35.01(c-7),40.02(c-8),50.24(c-9),37.13(c-10),32.16(c-11),70.74(c-12),49.26(c-13),51.72(c-14),31.42(c-15),26.41(c-16),50.76(c-17),16.22(c-18),16.72(c-19),73.32(c-20),21.89(c-21),43.56(c-22),18.69(c-23),44.04(c-24),69.74(c-25),30.12(c-26),29.89(c-27),28.07(c-28),15.82(c-29),17.36(c-30),171.99(-coo-),45.57(-ch2-nh-),44.74(-nh-ch2-),38.52(-ch-coo-),26.65(-ch2-),22.78(-ch2-)。
实施例24
细胞毒实验
实验目的:
通过体外培养人肺癌细胞a549,人胃癌细胞mgc-803,人胃癌细胞sgc-7901,人乳腺癌细胞mcf-7,人前列腺癌细胞pc-3,卵巢上皮细胞iose144,采用srb法检测细胞增殖活性,筛选受试化合物的体外抗肿瘤活性。
实验材料:
实验方法:
1.受试药物的配制:准确称取受试样品各2mg,用dmso配置成10mm,100μm的母液,用相应培养基配制成不同的浓度,0.22μm滤膜过滤备用。
2.细胞增殖抑制实验:取对数生长期细胞,调整密度为5×104加入不同浓度含药培养基100μl,对照组每孔加培养基100μl,对照组与药物组均设3个复孔,另设空白对照组,不加细胞只加培养基的作为对照组调零。将96孔板置5%co2培养箱(37℃)培养48h,加入0.04%srb20μl,继续培养4h后,弃上清,加入dmso150μl,振荡溶解10min,酶标仪于515nm处测定od值。
按公式计算抑制率,计算三次平行试验的ic50平均值,结果参见表1。
抑制率(%)=[(1-用药组平均od值/对照组平均od值)]×100%。
表1:化合物的细胞毒性
由表1中数据可知,本发明四环三萜衍生物对所测肿瘤细胞系均有不同程度的抑制细胞增长作用,抑制作用强于母体化合物。且对iose144无细胞毒活性。其中化合物17对所测肿瘤细胞系的活性最优(ic50=1.07±0.05μm至6.51±0.3μm),显著提升丙氨酸的活性,且显著优于对照丝裂霉素。
实施例25
将实施例1~实施例23分别进行阻滞和细胞凋亡,现选取最优实施例17进行详细说明。针对实施例17进行周期阻滞实验。
从图1~图4中,周期阻滞评估表明,相比对照组,经化合物17给药的a549细胞中,处于s期的细胞数目显著增多,提示化合物17能够诱导a549细胞s期阻滞。
实施例26
进行细胞凋亡实验。
如图5~图9所示,以及图10、图11,细胞凋亡实验结果显示,同母体化合物ad-2相比,实施例17对肺癌a549细胞具有显著的促进凋亡作用。
- 还没有人留言评论。精彩留言会获得点赞!